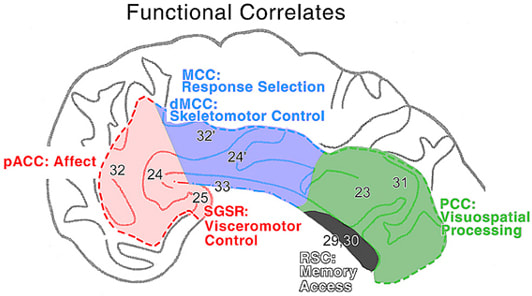
Cingulate Gyrus: Functional Correlations of the 4 Cingulate Regions
The neurobiological model of cingulate cortex requires that each cytoarchitectural area and region be associated with different functions or structure/function correlations. This does not imply no overlap in functions, rather, it means that each structurally distinct area makes a functionally unique contribution to brain activity. Identification of the cytoarchitectural border between areas 24 and 24' (Vogt et al., 1995) followed by its identification with functional imaging (Whalen et al., 1998; Bush et al., 1998) validates the importance of the neurobiological model based on structure/function correlations. Figure 1 presents the neurobiological model in the morphological context of four cingulate regions. As previously suggested (Vogt et al., 1997), pACC is involved in affect, SGSR in visceromotor control, MCC in response selection including dorsal MCC (dMCC) in skeletomotor control, PCC in visuospatial processing, and RSC in memory access.
Figure 1. The functional correlates are derived from electrical stimulation, stroke case reports, neuropsychological studies in monkey, and human functional imaging research. In addition to the four regions identified, there are two important subregions in pACC and MCC. These include the visceromotor control cortex in SGSR and the skeletomotor control subregion in dorsal MCC. The dMCC includes area 32' and the cingulate motor areas on the dorsal and ventral banks of the cingulate sulcus in this region of the cingulate gyrus.
Perigenual ACC and Subgenual Subregion in Affect
The perigenual areas are associated with affective experiences and are directly engaged in autonomic regulation. The term “direct” is crucial because it excludes general sensory and motor activities that do not have a specific role in autonomic regulation. Electrical stimulation of dorsal perigenual cortex in humans produces fear, pleasure, and agitation (Meyer et al., 1973). The most frequent response in this series was intense or overwhelming fear including one individual who reported the feeling that death was imminent. Electrical stimulation of different parts of ACC evokes different responses in epilepsy patients (Bancaud and Talairach, 1992). Stimulation of pACC produced the report, “I was afraid and my heart started to beat,” whereas stimulation of MCC evoked the report, “I felt something, as though I was going to leave.” The former report is of pure fear, while the latter is one of an early premotor planning with motivational characteristics. In another series of experiments, the SGSR had elevated blood flow when healthy women recalled sad experiences (George et al., 1995)and the dorsal pACC had elevated blood flow when subjects were involved in a face recognition task and the faces expressed emotional content (George et al., 1993). Finally, autonomic activity is a frequent correlate of emotional behaviors, and visceromotor changes are the most consistent responses evoked by electrical stimulation of areas 24 and 25. In humans these responses include increases and decreases in respiratory and cardiac rate and blood pressure, mydriasis, piloerection, and facial flushing (Pool, 1954; Escobedo et al., 1973; Talairach et al., 1973). Visceral responses include nausea, vomiting, epigastric sensation, salivation or bowel or bladder evacuation (Pool and Ransohoff, 1949; Lewin and Whitty, 1960; Meyer et al., 1973).
Perigenual ACC has important connections with structures that directly regulate autonomic activity. Area 25 projects to brainstem autonomic nuclei and has been termed the visceromotor control region by Neafsey et al. (1993). Projections of area 25 include those to the nucleus of the solitary tract, the dorsal motor nucleus of the vagus, and the sympathetic thoracic intermediolateral cell column. There are also projections to the periaqueductal gray and reciprocal connections with the amygdala and detailed references for each projection have been provided (Vogt et al., 1997) and each of these connections likely contribute to the role of pACC in affect and autonomic regulation.
The perigenual areas are associated with affective experiences and are directly engaged in autonomic regulation. The term “direct” is crucial because it excludes general sensory and motor activities that do not have a specific role in autonomic regulation. Electrical stimulation of dorsal perigenual cortex in humans produces fear, pleasure, and agitation (Meyer et al., 1973). The most frequent response in this series was intense or overwhelming fear including one individual who reported the feeling that death was imminent. Electrical stimulation of different parts of ACC evokes different responses in epilepsy patients (Bancaud and Talairach, 1992). Stimulation of pACC produced the report, “I was afraid and my heart started to beat,” whereas stimulation of MCC evoked the report, “I felt something, as though I was going to leave.” The former report is of pure fear, while the latter is one of an early premotor planning with motivational characteristics. In another series of experiments, the SGSR had elevated blood flow when healthy women recalled sad experiences (George et al., 1995)and the dorsal pACC had elevated blood flow when subjects were involved in a face recognition task and the faces expressed emotional content (George et al., 1993). Finally, autonomic activity is a frequent correlate of emotional behaviors, and visceromotor changes are the most consistent responses evoked by electrical stimulation of areas 24 and 25. In humans these responses include increases and decreases in respiratory and cardiac rate and blood pressure, mydriasis, piloerection, and facial flushing (Pool, 1954; Escobedo et al., 1973; Talairach et al., 1973). Visceral responses include nausea, vomiting, epigastric sensation, salivation or bowel or bladder evacuation (Pool and Ransohoff, 1949; Lewin and Whitty, 1960; Meyer et al., 1973).
Perigenual ACC has important connections with structures that directly regulate autonomic activity. Area 25 projects to brainstem autonomic nuclei and has been termed the visceromotor control region by Neafsey et al. (1993). Projections of area 25 include those to the nucleus of the solitary tract, the dorsal motor nucleus of the vagus, and the sympathetic thoracic intermediolateral cell column. There are also projections to the periaqueductal gray and reciprocal connections with the amygdala and detailed references for each projection have been provided (Vogt et al., 1997) and each of these connections likely contribute to the role of pACC in affect and autonomic regulation.
Perigenual ACC has important connections with structures that directly regulate autonomic activity. Area 25 projects to brainstem autonomic nuclei and has been termed the visceromotor control region by Neafsey et al. (1993). Projections of area 25 include those to the nucleus of the solitary tract, the dorsal motor nucleus of the vagus, and the sympathetic thoracic intermediolateral cell column. There are also projections to the periaqueductal gray and reciprocal connections with the amygdala and detailed references for each projection have been provided (Vogt et al., 1997) and each of these connections likely contribute to the role of pACC in affect and autonomic regulation.
The perigenual areas are associated with affective experiences and are directly engaged in autonomic regulation. The term “direct” is crucial because it excludes general sensory and motor activities that do not have a specific role in autonomic regulation. Electrical stimulation of dorsal perigenual cortex in humans produces fear, pleasure, and agitation (Meyer et al., 1973). The most frequent response in this series was intense or overwhelming fear including one individual who reported the feeling that death was imminent. Electrical stimulation of different parts of ACC evokes different responses in epilepsy patients (Bancaud and Talairach, 1992). Stimulation of pACC produced the report, “I was afraid and my heart started to beat,” whereas stimulation of MCC evoked the report, “I felt something, as though I was going to leave.” The former report is of pure fear, while the latter is one of an early premotor planning with motivational characteristics. In another series of experiments, the SGSR had elevated blood flow when healthy women recalled sad experiences (George et al., 1995)and the dorsal pACC had elevated blood flow when subjects were involved in a face recognition task and the faces expressed emotional content (George et al., 1993). Finally, autonomic activity is a frequent correlate of emotional behaviors, and visceromotor changes are the most consistent responses evoked by electrical stimulation of areas 24 and 25. In humans these responses include increases and decreases in respiratory and cardiac rate and blood pressure, mydriasis, piloerection, and facial flushing (Pool, 1954; Escobedo et al., 1973; Talairach et al., 1973). Visceral responses include nausea, vomiting, epigastric sensation, salivation or bowel or bladder evacuation (Pool and Ransohoff, 1949; Lewin and Whitty, 1960; Meyer et al., 1973).
Perigenual ACC has important connections with structures that directly regulate autonomic activity. Area 25 projects to brainstem autonomic nuclei and has been termed the visceromotor control region by Neafsey et al. (1993). Projections of area 25 include those to the nucleus of the solitary tract, the dorsal motor nucleus of the vagus, and the sympathetic thoracic intermediolateral cell column. There are also projections to the periaqueductal gray and reciprocal connections with the amygdala and detailed references for each projection have been provided (Vogt et al., 1997) and each of these connections likely contribute to the role of pACC in affect and autonomic regulation.
MCC in Response Selection
The MCC has a pattern of organization that is similar to pACC. Thus, pACC is primarily involved in affect and it implements emotionally relevant, autonomic activity directly through the projections of area 25 to the various brainstem autonomic motor nuclei. In a similar way, MCC has a general role in response selection based on the motivational relevance of particular behaviors, and it implements these behaviors through cingulospinal projections that arise in the cingulate motor areas. It is this fundamental dichotomy in which pACC regulates autonomic function and MCC regulates skeletomotor function that underpins the division of ACC into two parts.
Different functions have been ascribed to the MCC including attention-for-action (Posner et al., 1988), response selection (Corbetta et al., 1991; Bench et al., 1992; Paus et al., 1993), error detection (Gemba et al., 1986), competition monitoring (Carter et al., 1998), anticipation (Murtha et al., 1996), and working memory (Petit et al., 1998). Although it may be possible to accommodate all of these functions with a single model of onearea that incorporates many separate populations of neurons, the facts of cingulate cortex organization suggest these various functions represent the activity in at least two divisions of MCC. Thus, MCC itself is composed of two subregions; the dorsal MCC (dMCC) in sulcal cortex that extends onto the superior cingulate gyrus and another on the surface of the CG.
The dMCC areas in the cgs have very large layer Vb neurons (Braak, 1976), project to the spinal cord (Biber et al., 1978; Dum and Strick, 1993) and supplementrary and primary motor and limbic cortices (Morecraft and Van Hoesen, 1992; Van Hoesen et al., 1993) and account for the many skeletomotor responses evoked by electrical stimulation. These latter responses include gestures such as touching, kneading, rubbing or pressing the fingers or hands together, and lip puckering or sucking (Escobedo et al., 1973; Meyer et al., 1973; Talairach et al., 1973). These movements are often adapted to the environment, they can be modified by sensory stimuli, and at times, resisted. These areas contain neurons with premotor discharge properties (Shima et al., 1991) that are coded according to the changing reward properties of particular behaviors (Shima and Tanji, 1998). Functional imaging studies show altered blood flow in this region during sequences of finger apposition movements (Schlaug et al., 1994; Kwan et al., 2000) and it has been shown that altering the reward properties of particular outcomes elevates blood flow in dMCC (Bush et al., 2001).
The dMCC, however, is not limited to regulating discrete motor activities and functional imaging studies show that it has a major role in cognitive activity associated with the response selection process and not necessarily resulting in movement. Cognitive processes associated with divided attention (Corbetta et al., 1991), selecting verbs to novel lists of nouns (Raichle et al., 1994; Raichle, 2000), and Stroop interference tasks (Pardo et al., 1990; Bench et al., 1992) also elevate blood flow in dMCC. Furthermore, generation of verbs to novel lists of nouns not only elevates flow in MCC but, as the task is practiced, blood flow returns to baseline in MCC, it is decreased in pACC, and PCC and medial parietal area 7m show an increase in activity (Raichle, 2000). Thus, it appears that patterns of neuronal activity in cingulate cortex reflect the differential contributions of each region to different aspects of task acquisition and performance as well as cognitive processing in the absence of movement.
Different functions have been ascribed to the MCC including attention-for-action (Posner et al., 1988), response selection (Corbetta et al., 1991; Bench et al., 1992; Paus et al., 1993), error detection (Gemba et al., 1986), competition monitoring (Carter et al., 1998), anticipation (Murtha et al., 1996), and working memory (Petit et al., 1998). Although it may be possible to accommodate all of these functions with a single model of onearea that incorporates many separate populations of neurons, the facts of cingulate cortex organization suggest these various functions represent the activity in at least two divisions of MCC. Thus, MCC itself is composed of two subregions; the dorsal MCC (dMCC) in sulcal cortex that extends onto the superior cingulate gyrus and another on the surface of the CG.
The dMCC areas in the cgs have very large layer Vb neurons (Braak, 1976), project to the spinal cord (Biber et al., 1978; Dum and Strick, 1993) and supplementrary and primary motor and limbic cortices (Morecraft and Van Hoesen, 1992; Van Hoesen et al., 1993) and account for the many skeletomotor responses evoked by electrical stimulation. These latter responses include gestures such as touching, kneading, rubbing or pressing the fingers or hands together, and lip puckering or sucking (Escobedo et al., 1973; Meyer et al., 1973; Talairach et al., 1973). These movements are often adapted to the environment, they can be modified by sensory stimuli, and at times, resisted. These areas contain neurons with premotor discharge properties (Shima et al., 1991) that are coded according to the changing reward properties of particular behaviors (Shima and Tanji, 1998). Functional imaging studies show altered blood flow in this region during sequences of finger apposition movements (Schlaug et al., 1994; Kwan et al., 2000) and it has been shown that altering the reward properties of particular outcomes elevates blood flow in dMCC (Bush et al., 2001).
The dMCC, however, is not limited to regulating discrete motor activities and functional imaging studies show that it has a major role in cognitive activity associated with the response selection process and not necessarily resulting in movement. Cognitive processes associated with divided attention (Corbetta et al., 1991), selecting verbs to novel lists of nouns (Raichle et al., 1994; Raichle, 2000), and Stroop interference tasks (Pardo et al., 1990; Bench et al., 1992) also elevate blood flow in dMCC. Furthermore, generation of verbs to novel lists of nouns not only elevates flow in MCC but, as the task is practiced, blood flow returns to baseline in MCC, it is decreased in pACC, and PCC and medial parietal area 7m show an increase in activity (Raichle, 2000). Thus, it appears that patterns of neuronal activity in cingulate cortex reflect the differential contributions of each region to different aspects of task acquisition and performance as well as cognitive processing in the absence of movement.
Posterior Cingulate, Caudomedial, & Retrosplenial Cortices in Visuospatial/Memory Functions
Valenstein et al. (1987) presented a case with extensive anterograde and retrograde amnesia following removal of an arteriovenous malformation near the splenium and referred to the syndrome as retrosplenial amnesia. Since involvement of the fornix may have contributed to the presentation in this case, Parker and Gaffan (1997) placed massive cingulate cortical or anterior thalamic lesions in monkeys and tested object-in-place memory. Although cingulate lesions failed to show a significant deficit, there was substantial impairment in the anterior thalamic group. Since the anterior thalamic nuclei have as their primary projection the RSC (Vogt et al., 1987), it is surprising that no deficit was observed following cortical lesions. Explanations for the negative finding in the monkey are that the cortical lesions of RSC were incomplete due to the effort to remove the entire gyrus in anterior and posterior cortices and/or the task was not sensitive to a cortical impairment. Other evidence links these cingulate gyral areas to memory and visuospatial functions.
Working memory tasks elevate glucose metabolism in the anterior thalamic nuclei (Friedman et al., 1990), one of the highest levels of basal glucose metabolism in the monkey brain is in RSC, and glucose metabolism in RSC is elevated when performing a delayed-response task (Matsunami et al., 1989). We have compared the distribution of anterior thalamic inputs to RSC and basal glucose metabolism in the monkey and there is a striking correlation between both (Vogt et al., 1997). The tight link between anterior thalamic projections to RSC, high levels of basal glucose metabolism and its modulation in both structures suggest that the anterior thalamic/RSC system is a pivotal player in memory functions.
Working memory tasks elevate glucose metabolism in the anterior thalamic nuclei (Friedman et al., 1990), one of the highest levels of basal glucose metabolism in the monkey brain is in RSC, and glucose metabolism in RSC is elevated when performing a delayed-response task (Matsunami et al., 1989). We have compared the distribution of anterior thalamic inputs to RSC and basal glucose metabolism in the monkey and there is a striking correlation between both (Vogt et al., 1997). The tight link between anterior thalamic projections to RSC, high levels of basal glucose metabolism and its modulation in both structures suggest that the anterior thalamic/RSC system is a pivotal player in memory functions.
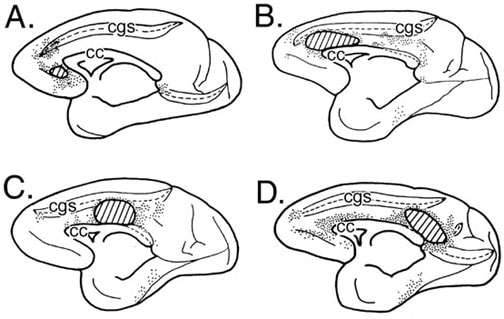
Figure 2. Four cases in which horseradish peroxidase was injected into the monkey cingulate gyrus and retrogradely labeled neurons plotted on the medial surface (each dot= 3-5 labeled neurons). The intracingulate connections support substantial interactions between pACC and PCC (A., B., D.), while the MCC (C.) tends to have much less interaction with either of these regions.
Area 23a and RSC are adjacent to each other and reciprocally connected (Vogt and Pandya, 1987). Moreover, relationships between perigenual and perisplenial cortices are profound. Area 23a is heavily labeled following a horseradish peroxidase injection into pACC (Fig. 2A) and pACC is labeled following injections of anterograde tracers into posterior area 23 (Pandya et al., 1981). Thus, the perigenual and perisplenial connection is reciprocal and involves only a small part of MCC following tracer injections into different parts of the CG (Fig. 2B-D). It appears the most profound intracingulate connections are reciprocal connections between pACC and PCC/CMSR. It is possible that activation of both of these regions with a common auditory input (Grasby et al., 1993) is strengthened by these reciprocal intracingulate connections. The linkage between pACC and the CMSR is even tighter when other cortical connections are considered. A limbic convergence zone in area 11m receives major inputs from both perigenual and perisplenial cortices with none from MCC (Carmichael and Price, 1995). Pericallosal cortex in both regions also receives direct subicular inputs that do not terminate appreciably in MCC (Rosene and Van Hoesen, 1977). Thus, sensory inputs, reciprocal intracingulate connections, and connections among limbic association areas preserves the functional integration of perigenual and perisplenial cortices to the exclusion of MCC.
The RSC and PCC are also involved in topographic and topokinetic memory. Olson et al. (1993) suggested that PCC is involved in large visual scene assessment, part of which is subserved by activity generated by the orbital position of the eye. Focal lesions that involve the right RSC impair memory of spatial positional relationships and are associated with topographic disorientation (Takahashi et al., 1997). Furthermore, mental navigation along memorized routes elevates blood flow in PCC (Berthoz, 1997; Ghaem et al., 1997; Maguire et al., 1998). Thus, PCS, CMSR, and RSC have many interconnections that subserve memory and visuospatial functions and their close relations to pACC suggest a coupling of the activity in these regions. It is possible, for example, that memory functions associated with emotional states are stored in pACC but their release for conscious consideration is mediated by the activity of RSC, PCC, and/or CMSR.
It appears we now have a general understanding of how each region of cingulate cortex contributes to brain function. What is not understood, however, defines a substantial void. We do not know the details of RSC function in primates because there are almost no studies showing unique activations in human and no single unit studies in monkey. It is not known how each of the various subfunctions that coactivate a region are related. For example, what is the relationship among groups of neurons that anticipate, code, and remember a noxious stimulus in MCC. The challenges for future research into cingulate cortex are large and numerous.
Area 23a and RSC are adjacent to each other and reciprocally connected (Vogt and Pandya, 1987). Moreover, relationships between perigenual and perisplenial cortices are profound. Area 23a is heavily labeled following a horseradish peroxidase injection into pACC (Fig. 2A) and pACC is labeled following injections of anterograde tracers into posterior area 23 (Pandya et al., 1981). Thus, the perigenual and perisplenial connection is reciprocal and involves only a small part of MCC following tracer injections into different parts of the CG (Fig. 2B-D). It appears the most profound intracingulate connections are reciprocal connections between pACC and PCC/CMSR. It is possible that activation of both of these regions with a common auditory input (Grasby et al., 1993) is strengthened by these reciprocal intracingulate connections. The linkage between pACC and the CMSR is even tighter when other cortical connections are considered. A limbic convergence zone in area 11m receives major inputs from both perigenual and perisplenial cortices with none from MCC (Carmichael and Price, 1995). Pericallosal cortex in both regions also receives direct subicular inputs that do not terminate appreciably in MCC (Rosene and Van Hoesen, 1977). Thus, sensory inputs, reciprocal intracingulate connections, and connections among limbic association areas preserves the functional integration of perigenual and perisplenial cortices to the exclusion of MCC.
The RSC and PCC are also involved in topographic and topokinetic memory. Olson et al. (1993) suggested that PCC is involved in large visual scene assessment, part of which is subserved by activity generated by the orbital position of the eye. Focal lesions that involve the right RSC impair memory of spatial positional relationships and are associated with topographic disorientation (Takahashi et al., 1997). Furthermore, mental navigation along memorized routes elevates blood flow in PCC (Berthoz, 1997; Ghaem et al., 1997; Maguire et al., 1998). Thus, PCS, CMSR, and RSC have many interconnections that subserve memory and visuospatial functions and their close relations to pACC suggest a coupling of the activity in these regions. It is possible, for example, that memory functions associated with emotional states are stored in pACC but their release for conscious consideration is mediated by the activity of RSC, PCC, and/or CMSR.
It appears we now have a general understanding of how each region of cingulate cortex contributes to brain function. What is not understood, however, defines a substantial void. We do not know the details of RSC function in primates because there are almost no studies showing unique activations in human and no single unit studies in monkey. It is not known how each of the various subfunctions that coactivate a region are related. For example, what is the relationship among groups of neurons that anticipate, code, and remember a noxious stimulus in MCC. The challenges for future research into cingulate cortex are large and numerous.
References
Bancaud, J. and Talairach, J. (1992). Clinical semiology of frontal lobe seizures. Adv. Neurol. 57, 3-58.
Bench, C.J., Frith, C.D., Grasby, P.M., Friston, K.J., Paulesu, E., Frackowiak, R.S.J., and Dolan, R.J. (1992). Patterns of cerebral activation during the Stroop colour word interference task: a positron emission tomography study. Neuropsychologia 31, 907-922.
Berthoz, A. (1997). Parietal and hippocampal contribution to topokinetic and topographic memory. Phil. Trans. Royal Soc. London, Ser.B. 352, 1437-1448.
Biber, M.P., Kneisley, L.W., and LaVail, J.H. (1978). Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp. Neurol. 59, 492-508.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109, 219-233.
Bush, G., Whalen, P.J., Rosen, B.R., Jenike, M.A., McInerney, S.C., and Rauch, S.L. (1998). The counting Stroop: An interference task specialized for functional neuroimaging-Validation study with functional MRI. Hum. Brain Map. 6, 270-282.
Bush, G., Vogt, B.A., Holmes, J., Dale, A.M., Greve, D., Jenike, M.A., and Rosen, B.R. (2001). Dorsal anterior cingulate cortex: A role in reward-based decision-making. Proc. Natl. Acad. Sci., in press.
Carmichael, S.T. and Price, J.L. (1995). Limbic Connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615-641.
Carter, C.S., Brauer, T.S., Barch, J.M., Botvinick, M.M., Noll. D., and Cohen, J.D. (1998). Anterior cingulate cortex, error detection, and on-line monitoring of performance. Science 280, 747-749.
Corbetta, M., Miezin, F.M., Dobmeyer, S., Shulman, G.L., and Petersen, S. E. (1991). Selective and divided attention during visual discrimination of shape, color, and speed: Functional anatomy by positron emission tomography. J. Neurosci. 11, 2383-2402.
Dum, R.P. and Strick, P.L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667-689.
Dum, R.P. and Strick, P.L. (1993). Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 415-441. Birkhäuser, Boston.
Escobedo, F., Fernández-Guardiola, A., and Solis, G. (1973). Chronic stimulation of the cingulum in humans with behavior disorders. In "Surgical Approaches in Psychiatry" (L. V. Laitinen and K. E. Livingston, Eds.), pp. 65-68. Lancaster (UK), MTP, Baltimore.
Friedman, H.R., Jana, J.D., and Goldman-Rakic, P.S. (1990). Enhancement of metabolic activity in the diencephalon of monkeys performing working memory tasks: A 2-deoxyglucose study in behaving rhesus monkeys. J. Cog. Neurosci. 2, 18-31.
Gemba, H., Sasaki, K., and Brooks, V.B. (1986). 'Error' potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosc. Let. 70, 223-227.
George, M.S., Ketter, T.A., Gill, D.S., Haxby, J. ., Ungerleider, L.G., Herscovitch, P., and Post, R.M. (1993). Brain regions involved in recognizing facial emotion or identity: An oxygen-15 PET study. J. Neuropsychiat. Clin. Neurosci. 5, 384-394.
George, M.S., Ketter, T.A., Parekh, P.I., Horwitz, B., Herscovitch, P., and Post, R.M. (1995). Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 152, 341-351.
Ghaem, O., Mellet, O., Crivello, F., Tzourio, N., Mazoyer, B., Berthoz, A., and Denis, M. (1997). Mental navigation along memorized routes activates the hippocampus, precuneus and insula. NeuroReport 8, 739-744.
Grasby, P.M., Frith, C.D., Friston, K.J., Bench, C., Frackowiak, R.S.J., and Dolan, R.J. (1993). Functional mapping of brain areas implicated in auditory-verbal memory function. Brain 116, 1-20.
Kwan, C.L., Crawley, A. P., Mikulis, D.J., and Davis, K.D. (2000). An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85, 359-374.
Lewin, W. and Whitty, C.W. . (1960). Effects of anterior cingulate stimulation in conscious human subjects. J. Neurophysiol. 23, 447.
Luppino, G., Matelli, M., Camarda, R.M., Gallese, V., and Rizzolatti, G. (1991). Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J. Comp. Neurol. 311, 463-482
Maguire, E.A., Frith, C.D., Burgess, N., Donnett, J.G., Pelaprat, D., and O'Keefe, J. (1998). Knowing where things are: Parahippocampal involvement in encoding object locations in
Matelli, M., Luppino, G., and Rizzolatti, G. (1991). Architecture of superior and mesial Area 6 and the adjacent cingulate cortex in the macaque monkey. J. Comp. Neurol. 311, 445-462.
Matsunami, K., Kawashima, T., and Satake, H. (1989). Mode of [14C] 2-deoxy-D-glucose uptake into retrosplenial cortex and other memory-related structures of the monkey during a delayed response. Brain Res. Bull. 22, 829 -838.
Meyer, G., McElhaney, M., Martin, W., and McGraw, C. P. (1973). Stereotactic cingulotomy with results of acute stimulation and serial psychological testing. In "Surgical Approaches in Psychiatry" (L. V. Laitinen and K. E. Livingston, Eds.), pp. 39-58. Lancaster (UK), MTP, Baltimore.
Morecraft, R.J. and Van Hoesen, G.W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in Areas 24c and 23c. J. Comp. Neurol. 322, 471-489.
Murtha, S., Chertkow, H., Beauregard, M., Dixon, R., Evans, A. (1996). Anticipation causes increased blood flow to the anterior cingulate cortex. Hum. Brain Mapp. 4, 103-112.
Neafsey, E.J., Terreberry, R.R., Hurley, K.M., Ruit, K.G., and Frysztak, R.J. (1993). Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 207-223. Birkhäuser, Boston.
Olson, C.R., Musil, S.Y., and Goldberg, M.E. (1993). Posterior cingulate cortex and visuospatial cognition: properties of single neurons in the behaving monkey. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 366-380. Birkhäuser Boston, Boston.
Pardo, J.V., Pardo, P.J., Janer, K.W., and Raichle, M. E. (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. USA 87, 256-259.
Parker, A. and Gaffan, D. (1997). The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia 35, 1093-1102.
Paus, T., Petrides, M., Evans, A.C., and Meyer, E. (1993). Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. J.Neurophysiol. 70, 453-469.
Petit, L., Courtney, S.M., Ungerleider, L.G., and Haxby, J.V. (1998). Sustained activity in the medial wall during working memory delays. J. Neurosci. 18, 9429-9437.
Pool, J.L. and Ransohoff, J. (1949). Autonomic effects on stimulating rostral portion of cingulate gyri in man. J. Neurophysiol. 12, 385-392.
Pool, J.L. (1954). The visceral brain of man. J. Neurosurg. 11, 45-63.
Posner, M.I., Peterson, S.E., Fox, P.T., and Raichle, M.E. (1988). Localization of cognitive operations in the human brain. Science 240, 1627-1631.
Raichle, M.E., Fiez, J.A., Videen, T.O., MacLeod, A.-M., Pardo, J.V., Fox, P.T., and Petersen, S.E. (1994). Practice-related changes in Alzheimer's disease. Cereb. Cortex 4, 8-26.
Raichle, M.E. (2000) The neural correlates of consciousness: An analysis of cognitive skill learning. In: “The New Cognitive Neurosciences” (M.S. Gazzaniga, Ed.), pp. 1305-1318, The MIT Press, Cambridge, MA.
Rosene, D.L. and Van Hoesen, G.W. (1977). Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science 198, 315-317
Schlaug, G., Knorr, U., and Seitz, R.J. (1994). Inter-subject variability of cerebral activations in acquiring a motor skill: A study with positron emission tomography. Exp. Brain Res. 98, 523-534.
Shima, K., Aya, K., Mushiake, H., Inase, M., Aizawa, H., and Tanji, J. (1991). Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J.Neurophysiol. 65, 188-202.
Shima, K. and Tanji, J. (1998). Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335-1338.
Takahashi, N., Kawamura, M., Shiota, J., Kasahata, N., and Hirayama, K. (1997). Pure topographic disorientation due to right retrosplenial lesion. Neurology 49, 464-469.
Talairach, J., Bancaud, J., Geier, S., Bordas-Ferrer, M., Bonis, A., and Szikla, G. (1973). The cingulate gyrus and human behavior. Electroencephalogr. Clin. Neurophysiol. 34, 45-52.
Valenstein, E., Bowers, D., Verfaellie, M., Heilman, K.M., Day, A., and Watson, R.T. (1987). Retrosplenial amnesia. Brain 110, 1631-1646.
Van Hoesen, G.W., Morecraft, R.J., and Vogt, B.A. (1993). Connections of the monkey cingulate cortex. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 249-284. Birkhäuser, Boston.
Vogt, B.A., Nimchinsky, E.A., Vogt, L.J., and Hof, P.R. (1995). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Vogt, B.A., Nimchinsky, E.A., and Hof, P.R. (1997). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Whalen, P.J., Bush, G., McNally, R.J., Wilhelm, S., McInerney, S.C., Jenike, M.A., and Rauch, S.L. (1998). The emotional counting Stroop paradigm: an fMRI probe of the anterior cingulate affective division. Biolog. Psychiatry 44, 1219-1228.
Bancaud, J. and Talairach, J. (1992). Clinical semiology of frontal lobe seizures. Adv. Neurol. 57, 3-58.
Bench, C.J., Frith, C.D., Grasby, P.M., Friston, K.J., Paulesu, E., Frackowiak, R.S.J., and Dolan, R.J. (1992). Patterns of cerebral activation during the Stroop colour word interference task: a positron emission tomography study. Neuropsychologia 31, 907-922.
Berthoz, A. (1997). Parietal and hippocampal contribution to topokinetic and topographic memory. Phil. Trans. Royal Soc. London, Ser.B. 352, 1437-1448.
Biber, M.P., Kneisley, L.W., and LaVail, J.H. (1978). Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp. Neurol. 59, 492-508.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109, 219-233.
Bush, G., Whalen, P.J., Rosen, B.R., Jenike, M.A., McInerney, S.C., and Rauch, S.L. (1998). The counting Stroop: An interference task specialized for functional neuroimaging-Validation study with functional MRI. Hum. Brain Map. 6, 270-282.
Bush, G., Vogt, B.A., Holmes, J., Dale, A.M., Greve, D., Jenike, M.A., and Rosen, B.R. (2001). Dorsal anterior cingulate cortex: A role in reward-based decision-making. Proc. Natl. Acad. Sci., in press.
Carmichael, S.T. and Price, J.L. (1995). Limbic Connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615-641.
Carter, C.S., Brauer, T.S., Barch, J.M., Botvinick, M.M., Noll. D., and Cohen, J.D. (1998). Anterior cingulate cortex, error detection, and on-line monitoring of performance. Science 280, 747-749.
Corbetta, M., Miezin, F.M., Dobmeyer, S., Shulman, G.L., and Petersen, S. E. (1991). Selective and divided attention during visual discrimination of shape, color, and speed: Functional anatomy by positron emission tomography. J. Neurosci. 11, 2383-2402.
Dum, R.P. and Strick, P.L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667-689.
Dum, R.P. and Strick, P.L. (1993). Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 415-441. Birkhäuser, Boston.
Escobedo, F., Fernández-Guardiola, A., and Solis, G. (1973). Chronic stimulation of the cingulum in humans with behavior disorders. In "Surgical Approaches in Psychiatry" (L. V. Laitinen and K. E. Livingston, Eds.), pp. 65-68. Lancaster (UK), MTP, Baltimore.
Friedman, H.R., Jana, J.D., and Goldman-Rakic, P.S. (1990). Enhancement of metabolic activity in the diencephalon of monkeys performing working memory tasks: A 2-deoxyglucose study in behaving rhesus monkeys. J. Cog. Neurosci. 2, 18-31.
Gemba, H., Sasaki, K., and Brooks, V.B. (1986). 'Error' potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosc. Let. 70, 223-227.
George, M.S., Ketter, T.A., Gill, D.S., Haxby, J. ., Ungerleider, L.G., Herscovitch, P., and Post, R.M. (1993). Brain regions involved in recognizing facial emotion or identity: An oxygen-15 PET study. J. Neuropsychiat. Clin. Neurosci. 5, 384-394.
George, M.S., Ketter, T.A., Parekh, P.I., Horwitz, B., Herscovitch, P., and Post, R.M. (1995). Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 152, 341-351.
Ghaem, O., Mellet, O., Crivello, F., Tzourio, N., Mazoyer, B., Berthoz, A., and Denis, M. (1997). Mental navigation along memorized routes activates the hippocampus, precuneus and insula. NeuroReport 8, 739-744.
Grasby, P.M., Frith, C.D., Friston, K.J., Bench, C., Frackowiak, R.S.J., and Dolan, R.J. (1993). Functional mapping of brain areas implicated in auditory-verbal memory function. Brain 116, 1-20.
Kwan, C.L., Crawley, A. P., Mikulis, D.J., and Davis, K.D. (2000). An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85, 359-374.
Lewin, W. and Whitty, C.W. . (1960). Effects of anterior cingulate stimulation in conscious human subjects. J. Neurophysiol. 23, 447.
Luppino, G., Matelli, M., Camarda, R.M., Gallese, V., and Rizzolatti, G. (1991). Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J. Comp. Neurol. 311, 463-482
Maguire, E.A., Frith, C.D., Burgess, N., Donnett, J.G., Pelaprat, D., and O'Keefe, J. (1998). Knowing where things are: Parahippocampal involvement in encoding object locations in
Matelli, M., Luppino, G., and Rizzolatti, G. (1991). Architecture of superior and mesial Area 6 and the adjacent cingulate cortex in the macaque monkey. J. Comp. Neurol. 311, 445-462.
Matsunami, K., Kawashima, T., and Satake, H. (1989). Mode of [14C] 2-deoxy-D-glucose uptake into retrosplenial cortex and other memory-related structures of the monkey during a delayed response. Brain Res. Bull. 22, 829 -838.
Meyer, G., McElhaney, M., Martin, W., and McGraw, C. P. (1973). Stereotactic cingulotomy with results of acute stimulation and serial psychological testing. In "Surgical Approaches in Psychiatry" (L. V. Laitinen and K. E. Livingston, Eds.), pp. 39-58. Lancaster (UK), MTP, Baltimore.
Morecraft, R.J. and Van Hoesen, G.W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in Areas 24c and 23c. J. Comp. Neurol. 322, 471-489.
Murtha, S., Chertkow, H., Beauregard, M., Dixon, R., Evans, A. (1996). Anticipation causes increased blood flow to the anterior cingulate cortex. Hum. Brain Mapp. 4, 103-112.
Neafsey, E.J., Terreberry, R.R., Hurley, K.M., Ruit, K.G., and Frysztak, R.J. (1993). Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 207-223. Birkhäuser, Boston.
Olson, C.R., Musil, S.Y., and Goldberg, M.E. (1993). Posterior cingulate cortex and visuospatial cognition: properties of single neurons in the behaving monkey. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 366-380. Birkhäuser Boston, Boston.
Pardo, J.V., Pardo, P.J., Janer, K.W., and Raichle, M. E. (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. USA 87, 256-259.
Parker, A. and Gaffan, D. (1997). The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia 35, 1093-1102.
Paus, T., Petrides, M., Evans, A.C., and Meyer, E. (1993). Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. J.Neurophysiol. 70, 453-469.
Petit, L., Courtney, S.M., Ungerleider, L.G., and Haxby, J.V. (1998). Sustained activity in the medial wall during working memory delays. J. Neurosci. 18, 9429-9437.
Pool, J.L. and Ransohoff, J. (1949). Autonomic effects on stimulating rostral portion of cingulate gyri in man. J. Neurophysiol. 12, 385-392.
Pool, J.L. (1954). The visceral brain of man. J. Neurosurg. 11, 45-63.
Posner, M.I., Peterson, S.E., Fox, P.T., and Raichle, M.E. (1988). Localization of cognitive operations in the human brain. Science 240, 1627-1631.
Raichle, M.E., Fiez, J.A., Videen, T.O., MacLeod, A.-M., Pardo, J.V., Fox, P.T., and Petersen, S.E. (1994). Practice-related changes in Alzheimer's disease. Cereb. Cortex 4, 8-26.
Raichle, M.E. (2000) The neural correlates of consciousness: An analysis of cognitive skill learning. In: “The New Cognitive Neurosciences” (M.S. Gazzaniga, Ed.), pp. 1305-1318, The MIT Press, Cambridge, MA.
Rosene, D.L. and Van Hoesen, G.W. (1977). Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science 198, 315-317
Schlaug, G., Knorr, U., and Seitz, R.J. (1994). Inter-subject variability of cerebral activations in acquiring a motor skill: A study with positron emission tomography. Exp. Brain Res. 98, 523-534.
Shima, K., Aya, K., Mushiake, H., Inase, M., Aizawa, H., and Tanji, J. (1991). Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J.Neurophysiol. 65, 188-202.
Shima, K. and Tanji, J. (1998). Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335-1338.
Takahashi, N., Kawamura, M., Shiota, J., Kasahata, N., and Hirayama, K. (1997). Pure topographic disorientation due to right retrosplenial lesion. Neurology 49, 464-469.
Talairach, J., Bancaud, J., Geier, S., Bordas-Ferrer, M., Bonis, A., and Szikla, G. (1973). The cingulate gyrus and human behavior. Electroencephalogr. Clin. Neurophysiol. 34, 45-52.
Valenstein, E., Bowers, D., Verfaellie, M., Heilman, K.M., Day, A., and Watson, R.T. (1987). Retrosplenial amnesia. Brain 110, 1631-1646.
Van Hoesen, G.W., Morecraft, R.J., and Vogt, B.A. (1993). Connections of the monkey cingulate cortex. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 249-284. Birkhäuser, Boston.
Vogt, B.A., Nimchinsky, E.A., Vogt, L.J., and Hof, P.R. (1995). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Vogt, B.A., Nimchinsky, E.A., and Hof, P.R. (1997). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Whalen, P.J., Bush, G., McNally, R.J., Wilhelm, S., McInerney, S.C., Jenike, M.A., and Rauch, S.L. (1998). The emotional counting Stroop paradigm: an fMRI probe of the anterior cingulate affective division. Biolog. Psychiatry 44, 1219-1228.