Cingulate Gyrus: Cytology of Cingulate Areas
A few overview comments of cingulate cytoarchitecture and histological preparations used for its analysis are in order before pursuing the details of each area’s organization. First, although the border between areas 24 and 23 is not marked with a consistent sulcus, this border is comprised of the most fundamental change from an agranular anterior cortex to the granular posterior cortex. The presence of a layer IV in posterior cingulate cortex suggests there is an intermediate level of cortical processing between thalamic and cortical afferents that does not occur in ACC. The anterior areas have thalamic and cortical projections directly to the basal dendrites of layer III and the apical dendrites of layer V pyramidal neurons; presumably for more rapid processing and output functions (Vogt and Peters, 1981). Second, a very substantial part of area 32 (i.e., about 50%) is in the rostral cingulate sulcus and, when present, the paracingulate sulcus. Third, the ectocallosal areas meet at midcingulate levels and are not separated by a long expanse of areas 23 and 24 as suggested by the Brodmann map. Fourth, the area on the cingulate gyrus with the thickest layer IV is area 31 around the splenial sulci. Finally, cortical transition thoughout the cingulate gyrus incorporates many different cytological changes including increases in neuron density in the rostrocaudal and ventrodorsal directions, progressively greater expression of nonphosphorylated neurofilament proteins in layer IIIc pyramids and their “predecessors” in deep layer III, and elaboration of the basal dendrites of pyramidal neurons particularly in layers III and V throughout the retrosplenial areas.
The cytology of the cingulate areas is considered here in a systematic manner beginning with the least differentiated ectocallosal areas and proceeding through the subgenual and dorsal anterior cingulate areas to the retrosplenial and posterior cingulate areas. This analysis builds on the work of Brodmann and Nissl-stained preparations, however, modern immunohistochemical techniques provide important new insights into the structure of each area. The antibody for neuron-specific nuclear binding protein (NeuN; Mullen et al., 1992) is an important tool because it labels neurons only and does not react with glial and vascular elements that add noise to the analysis of neuronal architectonics. In addition, the immunoreactivity is so intense that documentary photographs can have a high degree of contrast and the laminar architecture can be shown with great clarity at low magnifications which is not the case for Nissl-stained preparations. Another important antibody is SMI32 for nonphosphorylated neurofilament proteins (NFP-ir). This antibody has proven one of the most valuable markers of unique neuronal phenotypes and it identifies with great precision particular layers and, therefore, area borders. It is of most help in the granular layer of retrosplenial and ectosplenial cortices, layer IIIc in PCC, and layer Va throughout the entire cingulate gyrus including the cingulate motor areas in the depths of the cingulate sulcus. This antibody does not label neurons in layer II and most of layer IIIab, in which instance the NeuN antibody is more informative. These two antibodies provide many new insights into the structure of each cingulate area and Nissl stains are now of little value.
The cytology of the cingulate areas is considered here in a systematic manner beginning with the least differentiated ectocallosal areas and proceeding through the subgenual and dorsal anterior cingulate areas to the retrosplenial and posterior cingulate areas. This analysis builds on the work of Brodmann and Nissl-stained preparations, however, modern immunohistochemical techniques provide important new insights into the structure of each area. The antibody for neuron-specific nuclear binding protein (NeuN; Mullen et al., 1992) is an important tool because it labels neurons only and does not react with glial and vascular elements that add noise to the analysis of neuronal architectonics. In addition, the immunoreactivity is so intense that documentary photographs can have a high degree of contrast and the laminar architecture can be shown with great clarity at low magnifications which is not the case for Nissl-stained preparations. Another important antibody is SMI32 for nonphosphorylated neurofilament proteins (NFP-ir). This antibody has proven one of the most valuable markers of unique neuronal phenotypes and it identifies with great precision particular layers and, therefore, area borders. It is of most help in the granular layer of retrosplenial and ectosplenial cortices, layer IIIc in PCC, and layer Va throughout the entire cingulate gyrus including the cingulate motor areas in the depths of the cingulate sulcus. This antibody does not label neurons in layer II and most of layer IIIab, in which instance the NeuN antibody is more informative. These two antibodies provide many new insights into the structure of each cingulate area and Nissl stains are now of little value.
Ectocallosal Region
The ectocallosal region is composed of areas 33, 33', and 26. Brodmann showed that area 33 surrounds the rostral one-third of the corpus callosum beginning in the subgenual region where it abuts area 25 and continued around the corpus callosum to terminate dorsal to the genu. Although his map shows that area 26 surrounds only the most caudal end of the splenium and it did not abut area 33 rostrally, these observations cannot be verified histologically. Indeed, area 33 extends further caudally in the form of area 33’ and area 26 extends rostrally above the splenium and areas 33’ and 26 abut each other at midcingulate levels in the form of an undifferentiated cortex. The ectocallosal areas dorsal to the corpus callosum are shown in Figure 1.
Figure 1. Ectocallosal areas. The levels begin rostrally just dorsal to the genu and extend to a caudal level, the asterisks indicate layer Va, and the bracket in d. emphasizes that neurons in the deep part of layer III of area 33' are NFP-ir which serves as the basis for designating a caudal division of area 33. An undifferentiated area 29 appears in e and it appears that undifferentiated ectocallosal cortex continues along the full length of the CC.
Area 33 has a broad and poorly differentiated layers II/III, a layer V with a moderate number of NFP-ir pyramids and a layer VI of small neurons (Fig. 1a., b.). There is an ectocallosal area in the depths of the callosal sulcus at a midcingulate level (Fig. 1c., d.), but it has features that differentiate it from area 33. This area 33' has a more clearly laminated structure, layers II/III are thinner and contain somewhat smaller neurons, while the large neurons in the deep part of layer III are NFP-ir as is not the case for area 33 (compare Fig. 1 b. and c. in the brackets). Finally, a small number of layer V neurons are NFP-ir as is the case for area 33.
At a level just caudal to the midpoint in the corpus callosum (Figs. 1e), the external pyramidal layer contains small neurons with a granular appearance that suggest this is one of the most rostral levels of area 29. There is a striking NFP-ir in pyramids throughout layer III of areas 29 and 30, and layer Va has many more NFP-ir pyramids than is the case for either area 33 or 33'. It is interesting that at this rostral level of area 29, the neurons in the granular layer have not yet fully expressed NFP-ir like they do at caudal levels (Fig. 11). It is also difficult at this transitional level of Figure 1e to identify the features of either area 33' or area 26 and this poorly differentiated cortex lateral to area 29 is not designated in Figure 1e. It appears that rather than a loss of ectosplenial cortex between areas 33' and 26, there is a short distance of poorly differentiated and transitional cortex.
The granular layer of area 26 and its heavily NFP-ir pyramids are shown in Figure 1g. Area 26 has almost no deep layer which Braak (1979a) referred to this as essentially a layer VI of multiform neurons. The few deep lying pyramids that are present express NFP-ir. The differentiation and extension of areas 29 and 30 along the ventral bank of the cingulate gyrus is also seen in Figure 1, but is described in detail later as is the extension of area 26 around the splenium of the corpus callosum. Thus, the ectocallosal areas ring the fundus of the full rostrocaudal extent of the callosal sulcus. Only at a level just caudal to the callosal midpoint is there are short stretch of undifferentiated cortex that lacks the features of either areas 33' or 26.
Area 33 has a broad and poorly differentiated layers II/III, a layer V with a moderate number of NFP-ir pyramids and a layer VI of small neurons (Fig. 1a., b.). There is an ectocallosal area in the depths of the callosal sulcus at a midcingulate level (Fig. 1c., d.), but it has features that differentiate it from area 33. This area 33' has a more clearly laminated structure, layers II/III are thinner and contain somewhat smaller neurons, while the large neurons in the deep part of layer III are NFP-ir as is not the case for area 33 (compare Fig. 1 b. and c. in the brackets). Finally, a small number of layer V neurons are NFP-ir as is the case for area 33.
At a level just caudal to the midpoint in the corpus callosum (Figs. 1e), the external pyramidal layer contains small neurons with a granular appearance that suggest this is one of the most rostral levels of area 29. There is a striking NFP-ir in pyramids throughout layer III of areas 29 and 30, and layer Va has many more NFP-ir pyramids than is the case for either area 33 or 33'. It is interesting that at this rostral level of area 29, the neurons in the granular layer have not yet fully expressed NFP-ir like they do at caudal levels (Fig. 11). It is also difficult at this transitional level of Figure 1e to identify the features of either area 33' or area 26 and this poorly differentiated cortex lateral to area 29 is not designated in Figure 1e. It appears that rather than a loss of ectosplenial cortex between areas 33' and 26, there is a short distance of poorly differentiated and transitional cortex.
The granular layer of area 26 and its heavily NFP-ir pyramids are shown in Figure 1g. Area 26 has almost no deep layer which Braak (1979a) referred to this as essentially a layer VI of multiform neurons. The few deep lying pyramids that are present express NFP-ir. The differentiation and extension of areas 29 and 30 along the ventral bank of the cingulate gyrus is also seen in Figure 1, but is described in detail later as is the extension of area 26 around the splenium of the corpus callosum. Thus, the ectocallosal areas ring the fundus of the full rostrocaudal extent of the callosal sulcus. Only at a level just caudal to the callosal midpoint is there are short stretch of undifferentiated cortex that lacks the features of either areas 33' or 26.
Perigenual Areas
The subgenual subregion (SGSR) is comprised of areas 33, 25, and 24a and the caudal extensions of areas 32 and 12. Area 25 is the principal component of this region and is of particular interest because of its projections to autonomic brainstem nuclei and its role as a visceromotor control region (Neafsey et al., 1993). The architecture of area 25 differentiates directly from area 33 as shown in Figure 2A where the broad and undifferentiated pyramidal layer of area 33 abuts area 25 and a broad and relatively uniform layer II/III of medium-sized pyramids occurs. There is a slight intensification of layer Va in terms of both size and density of pyramidal neurons and a poorly differentiated layer VI. Small islands of layer II pyramidal neurons in caudal levels of area 25 have been observed (Vogt et al., 1997), however, this feature is variable. Figure 2 shows two levels through this subgenual region. It appears that the dorsal and rostral parts of this cortex have the aggregations of neurons in layer II. Since this clumping is not a characteristic of area 24, this cortex is termed rostral area 25 (25r). Braak (1979b) referred to this as area anterogenualis simplex. here also appears to be scattered clumping of layer II neurons in area 33 in this subgenual region.
Figure 2. Area 25 is the main component of the SGSR and its cytology is not uniform. The rostral part of area 25 (area 25r) is ventral to ectogenual area 33 and has some neuron aggregations in layer II (asterisks) and dense NFP-ir neuronal plexuses in layers III and V. Area 25c has a less differentiated structure with layers III and V having no subdivisions, and a modest number of NFP-ir neurons in layer V and few in layer III.
Caudal to area 25r is the area Brodmann termed area 25 that is shown in Figure 2A in “Cingulate Maps.” Figure 2B here shows that this area has a clear layer II that is negative for NFP-ir. It has a layer III of medium-sized pyramids that are sparsely immunoreactive for NFP, a layer V of slightly larger neurons that are also NFP-ir, and a layer VI of small neurons. Overall, this area is quite compact in comparison to area 25r. Since this area is caudal to area 25r, it is termed area 25c.
Areaanterogenual magnoganglionaris was identified by Braak (1979b) as a feature of cortex rostral to the genu. This provided one of the early arguments that area 24 does not surround the genu from its supracallosal position with a single architecture. Indeed, the broad layer Va of large pyramids that distinguishes this area 24b in perigenual ACC can be seen in Figures 3B (labeled area 24) and 10. Rostral and ventral to area 24b are areas 32 and 12. It is possible to identify components of areas 24a and 24b that have these magnocellular features and this is one of the primary distinctions between rostral and caudal divisions of area 24 (i.e., area 24 vs area 24').
A very accurate view of area 24 architecture is provided by NeuN and SMI32 preparations shown in Figure 3 with the gyral surface at a perigenual level. In the low magnification, orientation photograph, the primary differences between areas 24a and 24b can be seen; area 24a has a less dense layer II, thinner and less dense layer Va and a broad and less neuron dense layer VI when compared to area 24b. Each of these features are magnified where the subdivisions of layers V and VI are apparent as are a number of features of area 24b. The NeuN immunoreacted section shows an interesting feature for both areas 24a and 24b and, as noted below, for area 24' as well. Although layer III is not divisible with medium-sized neurons in the upper and large and NFP-ir neurons in the deeper part, this layer does have neuron dense superficial and sparse deep parts. This fact is noted in Figures 3, 5, and 6 with pairs of arrows spanning between the NeuN and SMI32 preparations.
Another important feature of area 24b is that it has many small neurons intermingled with the large ones in layer Va. The neurons in layer Va tend to form aggregates and they are NFP-ir. Layer Vb SMI32 immunostaining is magnified by a factor of two to show that neurons in this layer also form aggregates (asterisks) and they are NFP-ir. Among these layer Vb neurons are the NFP-ir, spindle neurons of Nimchinsky (Nimchinsky et al., 1995). These neurons are bipolar projection cells that are unique to limbic cortex including the insula and they are observed exclusively in human and great ape brains but not in monkeys (Nimchinsky et al., 1999). Finally, although layers Va and Vb are less neuron dense in area 24a, a similar clumping of NFP-ir pyramids is observed in both of these layers but it is most prominent in layer Vb including the presence of spindle neurons. These features of layer V distinguish area 24 from its caudal counterpart area 24'.
Caudal to area 25r is the area Brodmann termed area 25 that is shown in Figure 2A in “Cingulate Maps.” Figure 2B here shows that this area has a clear layer II that is negative for NFP-ir. It has a layer III of medium-sized pyramids that are sparsely immunoreactive for NFP, a layer V of slightly larger neurons that are also NFP-ir, and a layer VI of small neurons. Overall, this area is quite compact in comparison to area 25r. Since this area is caudal to area 25r, it is termed area 25c.
Areaanterogenual magnoganglionaris was identified by Braak (1979b) as a feature of cortex rostral to the genu. This provided one of the early arguments that area 24 does not surround the genu from its supracallosal position with a single architecture. Indeed, the broad layer Va of large pyramids that distinguishes this area 24b in perigenual ACC can be seen in Figures 3B (labeled area 24) and 10. Rostral and ventral to area 24b are areas 32 and 12. It is possible to identify components of areas 24a and 24b that have these magnocellular features and this is one of the primary distinctions between rostral and caudal divisions of area 24 (i.e., area 24 vs area 24').
A very accurate view of area 24 architecture is provided by NeuN and SMI32 preparations shown in Figure 3 with the gyral surface at a perigenual level. In the low magnification, orientation photograph, the primary differences between areas 24a and 24b can be seen; area 24a has a less dense layer II, thinner and less dense layer Va and a broad and less neuron dense layer VI when compared to area 24b. Each of these features are magnified where the subdivisions of layers V and VI are apparent as are a number of features of area 24b. The NeuN immunoreacted section shows an interesting feature for both areas 24a and 24b and, as noted below, for area 24' as well. Although layer III is not divisible with medium-sized neurons in the upper and large and NFP-ir neurons in the deeper part, this layer does have neuron dense superficial and sparse deep parts. This fact is noted in Figures 3, 5, and 6 with pairs of arrows spanning between the NeuN and SMI32 preparations.
Another important feature of area 24b is that it has many small neurons intermingled with the large ones in layer Va. The neurons in layer Va tend to form aggregates and they are NFP-ir. Layer Vb SMI32 immunostaining is magnified by a factor of two to show that neurons in this layer also form aggregates (asterisks) and they are NFP-ir. Among these layer Vb neurons are the NFP-ir, spindle neurons of Nimchinsky (Nimchinsky et al., 1995). These neurons are bipolar projection cells that are unique to limbic cortex including the insula and they are observed exclusively in human and great ape brains but not in monkeys (Nimchinsky et al., 1999). Finally, although layers Va and Vb are less neuron dense in area 24a, a similar clumping of NFP-ir pyramids is observed in both of these layers but it is most prominent in layer Vb including the presence of spindle neurons. These features of layer V distinguish area 24 from its caudal counterpart area 24'.
Figure 3. Perigenual areas 24a and 24b. Although both areas have a prominent layer Va and aggregations of NFP-ir neurons in layer Vb (asterisks in 2X pullout), layer Va is more dense in area 24b than it is in area 24a. Area 24b also has a dense layer II. Although layer III is not divisible based on neuron sizes, there are fewer neurons in the deeper part of this layer in both areas (two-headed arrows).
Midcingulate Gyral Areas
One of the primary features of MCC is the cingulate motor areas along the ventral bank of the cingulate sulcus. Although this region has extensive connections with motor systems including direct projections to the spinal cord, the sulcal and gyral areas are not independent and likely function jointly under certain circumstances. Pivotal to these interactions are direct and reciprocal connections identified in the monkey brain. Figure 4 shows reconstructions of retrograde and anterograde tracers into sulcal and gyral areas showing the massive and reciprocal nature of these connections. Injections of retrograde tracers into rostral and midcingulate cortex on the ventral bank of the cingulate sulcus (Fig. 4A, B) show greatest inputs from areas within the sulcal cortex but also substantial input from along the midcingulate, posterior cingulate and caudomedial cingulate cortices. It is interesting to note that anterograde tracer injections into gyral and sulcal areas show the greatest overall projections originating from the dorsal part of perigenual ACC (Fig. 4C). In contrast, injections into sulcal areas and midcingulate gyral cortex show a more limited projection pattern primarily dorsal to the corpus callosum and rostral parts of PCC (Fig. 4D) and often limited to midcingulate cortex itself (e.g., Fig. 4E, F). Thus, even though the gyral areas do not themselves have direct projections to the spinal cord and are not motor areas, the gyral areas are one step earlier in the premotor planning process; a processing stage that likely involves evaluation of the motivational consequences of particular motor outputs and, as such, is crucial in early premotor planning events. Although perigenual ACC has a much broader influence over the activity of the entire cingulate gyrus, the midcingulate gyral and sulcal areas are more tightly interconnected and influence much smaller units of cortex.
Figure 4. The cingulate motor areas on the ventral bank of the cingulate sulcus are heavily interconnected with gyral cortex as shown with retrograde labeling with Fast Blue (A, B) or horseradish peroxidase (C-F). The rostral cingulate motor area in area 24c' has heavy inputs from most of the cingulate gyrus, while the perigenual areas 24a and 24b have the greatest input from the SGSR. Also, the gyral MCC areas (i.e., 24a'/b') have greatest interconnections with the motor cortex of the MCC and almost none from perigenual ACC and the PCC/CMSR/RSC (D, E, F).
The main component of midcingulate cortex is area 24'. The midcingulate region is involved in response selection in the narrow and broad sense. In the narrow sense it refers to driving skeletomotor activity via the cingulate motor projections to the spinal cord. Even areas that do not have direct spinal projections like gyral areas 24a' and 24b' may play an important role in driving behavior. One example would be the role of the emotion fear in driving avoidance behaviors through anterior midcingulate cortex (aMCC).
Response selection in the broad sense refers to mental selection of options not requiring a behavior per se, anticipatory and preparatory activity, monitoring behavioral outcomes, and plastic changes induced by noxious (painful) stimulation. Each of these functions are discussed and referenced in "MCC in Response Selection."
In spite of the critical need to define the border between anterior and posterior parts of ACC in functional imaging studies, there are no objective methods to define this border with structural imaging and standardized atlases. This border can only be defined cytoarchitecturally and approximations made for the purposes of functional imaging. A comparison of the low magnification, orientation photographs in Figures 3 and 5 makes a number of obvious points about the structure of area 24 in perigenual ACC and area 24' in MCC. The MCC is thicker and has a much broader layers II, III, Va, Vb, and VIa. A closer viewing of area 24b' in Figure 5 shows that layer II neurons are in irregular groups rather than consistent islands like in area 25r. Although layer III does not have large pyramids in its deep aspect (i.e., no apparent layer IIIc), neurons are more sparse in the deep rather than superficial part of this layer and there are a few NFP-ir scattered throughout deep layer III (note two pairs of vertical arrows). There are, however, too few of these latter neurons to define a layer IIIc subdivision. Layer Va is broad and the neurons have a very heterogeneous size profile with the typically large and NFP-ir pyramids in addition to many small and medium-sized pyramids. Although layer Vb neurons are sparse and NFP-ir, they do not form obvious aggregations as in area 24b. Finally, layer VI is quite broad and about one-third of its small neurons are NFP-ir. Area 24a' has thinner and less neuron dense layers II, III, and Va and it has a similar profile of NFP-ir neurons in all layers including III, V, and VI.
A study by Nimchinsky et al. (1997) makes a number of important points about the border between areas 24 and 24' and that between areas 24' and 23. First, the distribution of NFP-ir neurons in a horizontal section through the cingulate gyrus showed three levels of immunnoreactivity. In perigenual ACC most staining was in layer Va, substantial staining in layer Vb, and none in layer III. In area 24' the staining in layer Va was more focused (i.e., less in layer Vb) and there were moderate levels in layer III. In area 23 the staining was most intense in layer IIIc, less in layer Va, even less in layer Vb/VI and none in layers II-IIIab. Second, an equally important differentiation could be made with a computer-generated map of the distribution of calretinin-ir pyramidal neurons. Most of these neurons are in the perigenual ACC and a moderate number in the rostral half of MCC, while there are none in area 23 of PCC. The observations of Nimchinsky et al. (1997), therefore, complement the SMI32 and NeuN preparations in coronal sections assessed in Figures 3, 5, 6, 7, and 8 and later those for area 23.
The main component of midcingulate cortex is area 24'. The midcingulate region is involved in response selection in the narrow and broad sense. In the narrow sense it refers to driving skeletomotor activity via the cingulate motor projections to the spinal cord. Even areas that do not have direct spinal projections like gyral areas 24a' and 24b' may play an important role in driving behavior. One example would be the role of the emotion fear in driving avoidance behaviors through anterior midcingulate cortex (aMCC).
Response selection in the broad sense refers to mental selection of options not requiring a behavior per se, anticipatory and preparatory activity, monitoring behavioral outcomes, and plastic changes induced by noxious (painful) stimulation. Each of these functions are discussed and referenced in "MCC in Response Selection."
In spite of the critical need to define the border between anterior and posterior parts of ACC in functional imaging studies, there are no objective methods to define this border with structural imaging and standardized atlases. This border can only be defined cytoarchitecturally and approximations made for the purposes of functional imaging. A comparison of the low magnification, orientation photographs in Figures 3 and 5 makes a number of obvious points about the structure of area 24 in perigenual ACC and area 24' in MCC. The MCC is thicker and has a much broader layers II, III, Va, Vb, and VIa. A closer viewing of area 24b' in Figure 5 shows that layer II neurons are in irregular groups rather than consistent islands like in area 25r. Although layer III does not have large pyramids in its deep aspect (i.e., no apparent layer IIIc), neurons are more sparse in the deep rather than superficial part of this layer and there are a few NFP-ir scattered throughout deep layer III (note two pairs of vertical arrows). There are, however, too few of these latter neurons to define a layer IIIc subdivision. Layer Va is broad and the neurons have a very heterogeneous size profile with the typically large and NFP-ir pyramids in addition to many small and medium-sized pyramids. Although layer Vb neurons are sparse and NFP-ir, they do not form obvious aggregations as in area 24b. Finally, layer VI is quite broad and about one-third of its small neurons are NFP-ir. Area 24a' has thinner and less neuron dense layers II, III, and Va and it has a similar profile of NFP-ir neurons in all layers including III, V, and VI.
A study by Nimchinsky et al. (1997) makes a number of important points about the border between areas 24 and 24' and that between areas 24' and 23. First, the distribution of NFP-ir neurons in a horizontal section through the cingulate gyrus showed three levels of immunnoreactivity. In perigenual ACC most staining was in layer Va, substantial staining in layer Vb, and none in layer III. In area 24' the staining in layer Va was more focused (i.e., less in layer Vb) and there were moderate levels in layer III. In area 23 the staining was most intense in layer IIIc, less in layer Va, even less in layer Vb/VI and none in layers II-IIIab. Second, an equally important differentiation could be made with a computer-generated map of the distribution of calretinin-ir pyramidal neurons. Most of these neurons are in the perigenual ACC and a moderate number in the rostral half of MCC, while there are none in area 23 of PCC. The observations of Nimchinsky et al. (1997), therefore, complement the SMI32 and NeuN preparations in coronal sections assessed in Figures 3, 5, 6, 7, and 8 and later those for area 23.
Figure 5. The rostral gyral areas 24a'/b' are generally thicker than their perigenual ACC counterparts, they have a thicker layer Va, and there are more small neurons in layer Va. There also are more small neurons in the deep part of layer III and this layer does not yet have large enough neurons to specify a layer IIIc. There are almost no NFP-ir in layers II-III but quite a number in layers Va and Vb.
Cingulate Sulcal Areas
Although it is well recognized that cortex in the depths of the cingulate sulcus projects to the spinal cord and has neurons with pre-movement discharges, some of which code for the reward properties associated with particular movements, there is not complete agreement over the number and distribution of each cingulate motor area. Dum and Strick (1991, 1993) identify three areas with one in area 24c (CMAr), one in area 23c (CMAv), and one in area “6c” (CMAd). Their rostral border for area 23 extends quite far rostrally and it is unclear that the area 6c region is a CMA per se, particularly since a unique set of projections to primary motor cortex has not been identified for it (Nimchinsky et al., 1996). Although Morecraft and Van Hoesen (1992, 1998) identify a CMA in area 23c, Luppino et al. (1991) and Rizzolatti et al. (1996) show no electrically evoked activity from area 23c caudal to area 24d. A monkey cortex with features similar to Braak’s primitive gigantopyramidal field appears to be part of the area 24d of Luppino et al. (1991), however, area 24d is more extensive than the primitive gigantopyramidal field, it forms most of the dorsal and ventral banks of the cingulate sulcus (Nimchinsky et al., 1996), and it extends more caudally in human as discussed below. One fact stands out from these observations; there is at least one, large cingulate motor area in area 24d.
Since Rizzolatti et al. (1996) showed significant movement activity following electrical stimulation of area 24c, it is possible that this area also contains a motor area. Morecraft et al. (1996) showed that a face representation occurs at a perigenual part of area 24c. Thus, there may be a somatotopically organized motor area in area 24c of perigenual ACC that either extends into area 24c' of MCC or there might be two separate motor areas in areas 24c and 24c'. The concept of at least one rostral and one caudal cingulate motor area is supported by Nimchinsky et al. (1996) who showed that the rostral one in area 24c has projections to primary motor cortex originating mainly in deep layers, while projections to the same area originate from both deep and superficial layers of area 24d. The number and topography of the cingulate motor areas will be resolved in the human brain with event-related functional magnetic resonance imaging techniques. The goal of this morphological consideration is to assure that a precise definition of each cortical area is available for such studies.
The observations made here are based on a photographic series through 25 levels of the entire length of the cingulate sulcus. Although we previously showed with Nissl preparations that area 24c in the cingulate sulcus was quite short in its rostrocaudal extent (Vogt et al., 1995), immunohistochemical preparations suggest that this area extends further caudal and two levels are shown in Figure 6. Area 24c has a thick layer II and a layer III with relatively uniform and medium-sized pyramids that thin out considerably towards its deeper part. Since there are no particularly large pyramidal neurons in deep layer III, a layer IIIc cannot be identified. Layer Va is quite dense with medium and large pyramidal neurons many of which are NFP-ir. Indeed, there is a rich plexus of NFP-ir dendrites throughout layer Va (Fig. 6, SMI32).
It is important to notice that the architecture of area 24c is not constant in its mediolateral extent. In the low magnification photographs (Fig. 6A, B) a pair of asterisks identifies those parts of layers Va and VI that have an elevated number of larger neurons than in adjacent cortex. The edge of one of these aggregates is shown at high magnification to the right of a pair of asterisks in Figure 6A (NeuN). Below layer Va there are also many neurons in layer Vb which is normally neuron sparse. It is certainly true that neurons in layer Vb are often NFP-ir, although there is not the fine plexus of dendrites seen in layer Va. The neurons in layer VI underneath the layer Va/b aggregations are also larger than normal.
It is possible the mediolateral differences in cytoarchitecture reflect the differential organization of subareas associated with somatotopic organization of the cortex. To the extent that this cortex may include a motor area, the largest layer V neurons may innervate neurons in the spinal cord that control the distal limb musculature. Even though the architecture of the second area photographed in Figure 6B is somewhat different than its rostral counterpart with smaller neurons and a generally lower level of NFP-ir in layer Va, notice in the lowest magnification photomicrograph that the clumps of neurons in layers Va-VI still occur and in an approximately equivalent mediolateral position to those noted in the previous section (Fig. 6B. Area 24c, NeuN; asterisks). Although there are minor changes along this rostral part of cingulate sulcal cortex, there is a common cytological motif including large neurons in layer Vb that are characteristic of a motor area. The proposal in monkey studies that area 24c contains a cingulate motor area (Morecraft et al., 1996; Nimchinsky et al., 1996) is confirmed for human. Whether or not a single motor area extends into area 24c' or the latter represents a separate area will require further investigation.
Since Rizzolatti et al. (1996) showed significant movement activity following electrical stimulation of area 24c, it is possible that this area also contains a motor area. Morecraft et al. (1996) showed that a face representation occurs at a perigenual part of area 24c. Thus, there may be a somatotopically organized motor area in area 24c of perigenual ACC that either extends into area 24c' of MCC or there might be two separate motor areas in areas 24c and 24c'. The concept of at least one rostral and one caudal cingulate motor area is supported by Nimchinsky et al. (1996) who showed that the rostral one in area 24c has projections to primary motor cortex originating mainly in deep layers, while projections to the same area originate from both deep and superficial layers of area 24d. The number and topography of the cingulate motor areas will be resolved in the human brain with event-related functional magnetic resonance imaging techniques. The goal of this morphological consideration is to assure that a precise definition of each cortical area is available for such studies.
The observations made here are based on a photographic series through 25 levels of the entire length of the cingulate sulcus. Although we previously showed with Nissl preparations that area 24c in the cingulate sulcus was quite short in its rostrocaudal extent (Vogt et al., 1995), immunohistochemical preparations suggest that this area extends further caudal and two levels are shown in Figure 6. Area 24c has a thick layer II and a layer III with relatively uniform and medium-sized pyramids that thin out considerably towards its deeper part. Since there are no particularly large pyramidal neurons in deep layer III, a layer IIIc cannot be identified. Layer Va is quite dense with medium and large pyramidal neurons many of which are NFP-ir. Indeed, there is a rich plexus of NFP-ir dendrites throughout layer Va (Fig. 6, SMI32).
It is important to notice that the architecture of area 24c is not constant in its mediolateral extent. In the low magnification photographs (Fig. 6A, B) a pair of asterisks identifies those parts of layers Va and VI that have an elevated number of larger neurons than in adjacent cortex. The edge of one of these aggregates is shown at high magnification to the right of a pair of asterisks in Figure 6A (NeuN). Below layer Va there are also many neurons in layer Vb which is normally neuron sparse. It is certainly true that neurons in layer Vb are often NFP-ir, although there is not the fine plexus of dendrites seen in layer Va. The neurons in layer VI underneath the layer Va/b aggregations are also larger than normal.
It is possible the mediolateral differences in cytoarchitecture reflect the differential organization of subareas associated with somatotopic organization of the cortex. To the extent that this cortex may include a motor area, the largest layer V neurons may innervate neurons in the spinal cord that control the distal limb musculature. Even though the architecture of the second area photographed in Figure 6B is somewhat different than its rostral counterpart with smaller neurons and a generally lower level of NFP-ir in layer Va, notice in the lowest magnification photomicrograph that the clumps of neurons in layers Va-VI still occur and in an approximately equivalent mediolateral position to those noted in the previous section (Fig. 6B. Area 24c, NeuN; asterisks). Although there are minor changes along this rostral part of cingulate sulcal cortex, there is a common cytological motif including large neurons in layer Vb that are characteristic of a motor area. The proposal in monkey studies that area 24c contains a cingulate motor area (Morecraft et al., 1996; Nimchinsky et al., 1996) is confirmed for human. Whether or not a single motor area extends into area 24c' or the latter represents a separate area will require further investigation.
Figure 6. Area 24c in the rostral cingulate sulcus shown for two levels because this area may extend further caudally than earlier thought. In the low magnification photographs there are two aggregates of large neurons in layers Va and VI. The NeuN section in A that is magnified further shows the border with one of these clumps with a pair of asterisks around layer Va and once again at the highest magnification. It is likely that these intra-areal modulations in architecture reflect differential innervation patterns for parts of the skeletomotor projection system. The distinction between layers Va and Vb are difficult and many of these large neurons are NFP-ir. In this area, layer III not only is sparse in its deeper division but there are some large NFP-ir neurons therein, although an overt layer of large IIIc pyramids cannot be detected.
Area 24c' of MCC is cingulate motor cortex and is significantly thicker than its rostral counterpart. A number of significant changes occur in this cortex as shown in Figure 7. Layer III has many more NFP-ir neurons and, relatively speaking, there are fewer in layer V. Indeed, the dense plexus of dendrites characteristic of layer Va in area 24c is not present. The neurons in layer Va are generally smaller, while those in layer Vb are quite large and densely packed. In accordance with the rostrocaudal trend in area 24 toward layer III neurons expressing higher levels of NFP-ir in the caudal regions, the posterior of two levels of SMI32 shown in Figure 7B has a higher density of larger layer III neurons expressing NFP-ir. The rostrocaudal trend toward increased numbers of NFP-ir neurons in layer III has been shown for both sulcal (Nimchinsky et al., 1996) and gyral (Nimchinsky et al., 1997) cortex with full length, horizontal sections.
Area 24c' of MCC is cingulate motor cortex and is significantly thicker than its rostral counterpart. A number of significant changes occur in this cortex as shown in Figure 7. Layer III has many more NFP-ir neurons and, relatively speaking, there are fewer in layer V. Indeed, the dense plexus of dendrites characteristic of layer Va in area 24c is not present. The neurons in layer Va are generally smaller, while those in layer Vb are quite large and densely packed. In accordance with the rostrocaudal trend in area 24 toward layer III neurons expressing higher levels of NFP-ir in the caudal regions, the posterior of two levels of SMI32 shown in Figure 7B has a higher density of larger layer III neurons expressing NFP-ir. The rostrocaudal trend toward increased numbers of NFP-ir neurons in layer III has been shown for both sulcal (Nimchinsky et al., 1996) and gyral (Nimchinsky et al., 1997) cortex with full length, horizontal sections.
Figure 7. Two levels of area 24c' show this area has a thick layer Va that is parvicellular. There are so many neurons in layers III-VI that are NFP-ir that the SMI32 preparations look almost homogeneous. The large though not gigantic layer Vb pyramids are quite clear in both preparations and the NeuN (A) emphasizes the motor nature of this cortex.
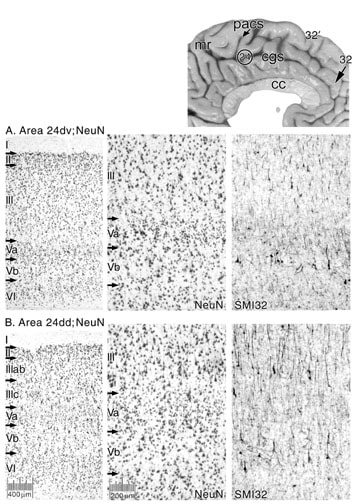
Area 24d contains the caudal cingulate motor area. The caudal border of area 24d is located somewhat anterior to the inflection point of the marginal ramus from the cingulate sulcus. There are very large pyramidal neurons in the deep part of layer V (Braak, 1976) and these are NFP-ir (Nimchinsky et al., 1996). Figure 8 shows the neuronal composition of area 24d. Area 24d has a broad layer II, relatively homogeneous layer III, deep layer Vb with the large pyramids and a layer VI. There are two unique aspects to layer V in area 24d. First, layer Va is parvicellular because it contains many small pyramids intermingled with larger neurons (Fig. 8). This structure is not readily apparent in Nissl-stained preparations because of co-staining of glial cells. In Figure 8 it is quite clear that layer Va has many small neurons in the NeuN preparation and that it also has a dense plexus of NFP-ir dendrites and small neurons in the SMI32 preparation. Second, layer Vb contains the gigantopyramidal neurons of Braak that are NFP-ir and aggregations of these neurons in layer Vb are particularly apparent in the SMI32 preparation of area 24d on the ventral bank of the sulcus (i.e., area 24dv). In addition, many of the large pyramids in layer III are NFP-ir, although a layer IIIc cannot be defined on the basis of neuronal size in comparison to superficial parts of layer III.
One of the striking outcomes of the present analysis with NeuN and SMI32 preparations is the fact that cortex on the dorsal (area 24dd) and ventral (area 24dv) banks of the cingulate sulcus share adequate similarities to be part of area 24d. This was not readily apparent in earlier studies of Nissl preparations (Vogt et al., 1995). The conclusion that area 24d extends onto the superior bank of the cingulate sulcus means that the short extension of area 32' previously noted on the dorsal bank at this caudal level has been displaced. In this situation, area 32' inserts mainly between areas 24c' and area 6aß.
There are some interesting differences between areas 24dd and 24dv that could have important functional consequences. First, area 24dd has more larger and NFP-ir neurons in layer III and these neurons reach the point where a layer IIIc is defined. This is an important transitional feature to area 32' where layer III pyramids are a significant characteristic. Second, layer Va in area 24dd is much thicker than in area 24dv, although it appears to contain the same overall proportions of small and large pyramids. Finally, layer Vb has slightly larger pyramids and they are solitary rather in groups as in area 24dv.
Area 24d contains the caudal cingulate motor area. The caudal border of area 24d is located somewhat anterior to the inflection point of the marginal ramus from the cingulate sulcus. There are very large pyramidal neurons in the deep part of layer V (Braak, 1976) and these are NFP-ir (Nimchinsky et al., 1996). Figure 8 shows the neuronal composition of area 24d. Area 24d has a broad layer II, relatively homogeneous layer III, deep layer Vb with the large pyramids and a layer VI. There are two unique aspects to layer V in area 24d. First, layer Va is parvicellular because it contains many small pyramids intermingled with larger neurons (Fig. 8). This structure is not readily apparent in Nissl-stained preparations because of co-staining of glial cells. In Figure 8 it is quite clear that layer Va has many small neurons in the NeuN preparation and that it also has a dense plexus of NFP-ir dendrites and small neurons in the SMI32 preparation. Second, layer Vb contains the gigantopyramidal neurons of Braak that are NFP-ir and aggregations of these neurons in layer Vb are particularly apparent in the SMI32 preparation of area 24d on the ventral bank of the sulcus (i.e., area 24dv). In addition, many of the large pyramids in layer III are NFP-ir, although a layer IIIc cannot be defined on the basis of neuronal size in comparison to superficial parts of layer III.
One of the striking outcomes of the present analysis with NeuN and SMI32 preparations is the fact that cortex on the dorsal (area 24dd) and ventral (area 24dv) banks of the cingulate sulcus share adequate similarities to be part of area 24d. This was not readily apparent in earlier studies of Nissl preparations (Vogt et al., 1995). The conclusion that area 24d extends onto the superior bank of the cingulate sulcus means that the short extension of area 32' previously noted on the dorsal bank at this caudal level has been displaced. In this situation, area 32' inserts mainly between areas 24c' and area 6aß.
There are some interesting differences between areas 24dd and 24dv that could have important functional consequences. First, area 24dd has more larger and NFP-ir neurons in layer III and these neurons reach the point where a layer IIIc is defined. This is an important transitional feature to area 32' where layer III pyramids are a significant characteristic. Second, layer Va in area 24dd is much thicker than in area 24dv, although it appears to contain the same overall proportions of small and large pyramids. Finally, layer Vb has slightly larger pyramids and they are solitary rather in groups as in area 24dv.
Figure 8. Since the dorsal and ventral banks of the cingulate sulcus at this caudal level represent different parts of a somatotopically organized motor field, an area 24dv (ventral) and 24dd (dorsal) were analyzed. Area 24d in these two locations represents an enlargement of this field from previous studies that relied on Nissl staining only (Vogt et al., 1995). The ventral bank has the hallmark aggregations of very large pyramidal neurons that are NFP-ir in layer Vb, while this layer in the dorsal division has solitary pyramids. There are also many more large and NFP-ir neurons in layer IIIc of area 24dd than in area 24dv.
The different morphologies of these two divisions of area 24d may relate to their differential involvement in skeletomotor and cognitive functions. Area 24dd mainly regulates muscles of the hindlimb and lower trunk, while area 24dv regulates mainly forelimb and upper trunk (Rizzolatti et al., 1996). Figure 8 shows that area 24dv on the ventral bank has a thin layer Va and somewhat smaller NFP-ir large neurons in layer Vb. In contrast, area 24dd has a much broader layer Va and larger layer Vb neurons that are most often solitary rather than in cellular aggregates.
The different morphologies of these two divisions of area 24d may relate to their differential involvement in skeletomotor and cognitive functions. Area 24dd mainly regulates muscles of the hindlimb and lower trunk, while area 24dv regulates mainly forelimb and upper trunk (Rizzolatti et al., 1996). Figure 8 shows that area 24dv on the ventral bank has a thin layer Va and somewhat smaller NFP-ir large neurons in layer Vb. In contrast, area 24dd has a much broader layer Va and larger layer Vb neurons that are most often solitary rather than in cellular aggregates.
Areas 32, 32', 6aα and 6aß
Area 32 is a rostral, dorsal and anterior division of cingulate cortex that forms a belt around area 24 (Brodmann, 1909). Although parts of it have been activated in a number of functional imaging studies associated with response selection such as generation of verbs to novel lists of nouns (Raichle et al., 1994) and Stroop interference tasks (Pardo et al., 1990; Bench et al., 1992), its specific contributions to brain function are not fully understood. Indeed, this stretch of cortex lies between the cingulate motor areas ventrally and medial frontal areas dorsally including the supplementary motor areas. It is reasonable to expect that this intermediate area plays a role in joining the functional activity of a number of frontal and cingulate motor areas. From a structural perspective, areas 32 and 32' have features that are transitional to granular prefrontal cortex. In perigenual ACC, area 32 is transitional from area 24 to areas 12, 10, and 9, while area 32' is transitional at dorsal levels to area 9 and rostral area 6aα. von Economo and Koskinas (1925; Fig. 1A in Cingulate Maps) emphasized these transitional positions with adjacent frontal areas in their designations FhL, Fel, Fdl, and Fcl, although they did not recognize an area equivalent to area 32'. Sarkisov et al. (1955) observed a transition area they called area 24/32 and 32/31. It is this cortex between areas 24c' and 6aα that is termed area 32'.
Area 32 has a well developed layer IIIc with most large pyramids being NFP-ir (Fig. 9A). Since these are the largest neurons in area 32, this inversion is a key transitional feature with area 24 where the largest and NFP-ir neurons are located in layer V. Layer IV is another transitional feature which contrasts sharply with the agranular areas 25 and 24. In area 32, layer IV is thin but continuous and is distinguished from the dysgranular organization of area 32' where there are clear interruptions in layer IV. Finally, layers V and VI are unremarkable, although layer Vb seems to have more neurons than is usual in area 24.
Area 32 has a well developed layer IIIc with most large pyramids being NFP-ir (Fig. 9A). Since these are the largest neurons in area 32, this inversion is a key transitional feature with area 24 where the largest and NFP-ir neurons are located in layer V. Layer IV is another transitional feature which contrasts sharply with the agranular areas 25 and 24. In area 32, layer IV is thin but continuous and is distinguished from the dysgranular organization of area 32' where there are clear interruptions in layer IV. Finally, layers V and VI are unremarkable, although layer Vb seems to have more neurons than is usual in area 24.
Figure 9. The cingulofrontal transition areas 32 and 32' are located in the perigenual ACC and MCC, respectively. Area 32 has a clear layer IV and large and heavily NFP-ir layer IIIc pyramids. These are the characteristics of association areas in general. At caudal levels of area 32, i.e., area 32', a region Brodmann did not label area 32, there is still a layer IV but it is dysgranular because there are places where neurons in layers IIIc and Va abut each other as noted with the pair of asterisks in NeuN preparations (B).
Area 32' of MCC inserts itself primarily between the supplementary motor area 6ab and area 24c'. This statement is a modification of a previous report that had a short extension of his area interposed between areas 24d and 6aa. Area 32' has a number of differences with area 32 in perigenual ACC. Area 32' has large layer IIIc pyramids that are NFP-ir, however, there are many fewer of these neurons (compare SMI32 for both in Fig.9). Although there are fewer large layer IIIc pyramids, the overall neuron density in layer IIIc is lower than in area 32. Layer IV is more often interrupted by bridges of neurons requiring the designation of dysgranular as reported previously and shown with Nissl preparations (Vogt et al., 1995). In Figure 9 a level of this area was photographed in which there was one such interruption at the pair of asterisks (Fig. 9B. Area 32'; NeuN). Finally, although layers Va and Vb can be differentiated with large neurons in the former, layer Vb has a very significant population of small and medium-sized neurons; i.e., it is not neuron sparse as in many of its ventral counterparts. Although it is true that layer Vb in area 32 has neuron sparse places, it also has a relatively high density of neurons. This also contrasts with area 24d which has a more typically neuron-sparse layer Vb.
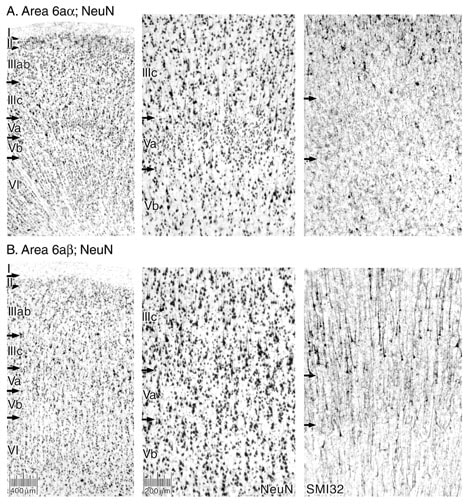
Dorsal and caudal to area 32' are the supplementary motor areas. Understanding these areas is crucial to the organization of the medial cortex motor areas and the culmination of a transition from area 24 to area 6. Matelli et al. (1991) defined the structure of F3 (area 6aα; Vogt and Vogt, 1919) and F6 (area 6ab) in monkey and the properties of electrically evoked activity have been described for these areas and the adjacent cingulate motor region (Luppino et al., 1991; Rizzolatti et al., 1996). Area F3 has large pyramids in deep layer III and layer Vb, while F6 has more uniform neurons in layer III, no clear demarcations within layer V including a lack of the very large layer Vb pyramids, and a layer VI that is much more dense than in its rostral counterpart. Similar organization has been reported with Nissl preparations in human (Zilles et al., 1996), however, these areas have not been considered in NeuN preparations and directly compared to those for SMI32. They areas form a critical boundary of the cingulate cortex and, as such, they represent a final stage of transition from area 32'.
Area 6aα has large layer Vb pyramids most of which are NFP-ir (Fig. 10). Layer III tends to have larger deep lying neurons than in the monkey and not as many are NFP-ir as in area 6ab. Area 6aα also has a minor population of small neurons in the superficial aspect of layer Va that is not noticeable in Nissl preparations. Comparison of this area with area 6ab in Figure 10 shows there are no small neurons at the superifical part of layer Va in the latter area, the large pyramids in layer IIIc are heavily NFP-ir and the distinction between layers Va and Vb is weak because of the elevated density of neurons in layers Vb and VI.
Comparisons among the subdivisions of area 6 and their “predecessor” area 32' on the cingulate gyrus include the following observations. Area 32' has more NFP-ir neurons in layer IIIc than does area 6aα, while area 6ab has many more such neurons in layer IIIc. Layer IV is dysgranular in area 32', while none exists in area 6, although a few small pyramids are present in the superficial part of layer Va that do not constitute a layer IV. Finally, layer V is quite similar in each of these areas in terms of neuron sizes, densities, and extent of NFP expression. In conclusion, it appears that area 32' shares many features with area 6aα, while area 32 is more closely aligned in overall morphology with that of area 6ab particularly in terms of the organization of layer III, although there is no layer IV in the latter area. Although area 32' has not been implicated as a cingulate motor area per se, it shares so many features of the supplementary motor areas that its role in some very closely aligned functions must be considered.
Area 32' of MCC inserts itself primarily between the supplementary motor area 6ab and area 24c'. This statement is a modification of a previous report that had a short extension of his area interposed between areas 24d and 6aa. Area 32' has a number of differences with area 32 in perigenual ACC. Area 32' has large layer IIIc pyramids that are NFP-ir, however, there are many fewer of these neurons (compare SMI32 for both in Fig.9). Although there are fewer large layer IIIc pyramids, the overall neuron density in layer IIIc is lower than in area 32. Layer IV is more often interrupted by bridges of neurons requiring the designation of dysgranular as reported previously and shown with Nissl preparations (Vogt et al., 1995). In Figure 9 a level of this area was photographed in which there was one such interruption at the pair of asterisks (Fig. 9B. Area 32'; NeuN). Finally, although layers Va and Vb can be differentiated with large neurons in the former, layer Vb has a very significant population of small and medium-sized neurons; i.e., it is not neuron sparse as in many of its ventral counterparts. Although it is true that layer Vb in area 32 has neuron sparse places, it also has a relatively high density of neurons. This also contrasts with area 24d which has a more typically neuron-sparse layer Vb.
Dorsal and caudal to area 32' are the supplementary motor areas. Understanding these areas is crucial to the organization of the medial cortex motor areas and the culmination of a transition from area 24 to area 6. Matelli et al. (1991) defined the structure of F3 (area 6aα; Vogt and Vogt, 1919) and F6 (area 6ab) in monkey and the properties of electrically evoked activity have been described for these areas and the adjacent cingulate motor region (Luppino et al., 1991; Rizzolatti et al., 1996). Area F3 has large pyramids in deep layer III and layer Vb, while F6 has more uniform neurons in layer III, no clear demarcations within layer V including a lack of the very large layer Vb pyramids, and a layer VI that is much more dense than in its rostral counterpart. Similar organization has been reported with Nissl preparations in human (Zilles et al., 1996), however, these areas have not been considered in NeuN preparations and directly compared to those for SMI32. They areas form a critical boundary of the cingulate cortex and, as such, they represent a final stage of transition from area 32'.
Area 6aα has large layer Vb pyramids most of which are NFP-ir (Fig. 10). Layer III tends to have larger deep lying neurons than in the monkey and not as many are NFP-ir as in area 6ab. Area 6aα also has a minor population of small neurons in the superficial aspect of layer Va that is not noticeable in Nissl preparations. Comparison of this area with area 6ab in Figure 10 shows there are no small neurons at the superifical part of layer Va in the latter area, the large pyramids in layer IIIc are heavily NFP-ir and the distinction between layers Va and Vb is weak because of the elevated density of neurons in layers Vb and VI.
Comparisons among the subdivisions of area 6 and their “predecessor” area 32' on the cingulate gyrus include the following observations. Area 32' has more NFP-ir neurons in layer IIIc than does area 6aα, while area 6ab has many more such neurons in layer IIIc. Layer IV is dysgranular in area 32', while none exists in area 6, although a few small pyramids are present in the superficial part of layer Va that do not constitute a layer IV. Finally, layer V is quite similar in each of these areas in terms of neuron sizes, densities, and extent of NFP expression. In conclusion, it appears that area 32' shares many features with area 6aα, while area 32 is more closely aligned in overall morphology with that of area 6ab particularly in terms of the organization of layer III, although there is no layer IV in the latter area. Although area 32' has not been implicated as a cingulate motor area per se, it shares so many features of the supplementary motor areas that its role in some very closely aligned functions must be considered.
Figure 10. Transition from area 32' to the caudal supplementary motor region (area 6aα) is characterized by large layer IIIc pyramids that are heavily NFP-ir and granularity of layer Va. Layer Vb is cell sparse and the neurons in layer Va express only low levels of NFP-ir. Area 6ab is adjacent to area 32 and, although a layer IV is not present, there are many small neurons intermingled with the large and NFP-ir pyramids in layer IIIc. Layer Vb is very neuron dense and these cells express only a low level of NFP-ir.
Ectosplenial and Retrosplenial Cortices
Each section through RSC includes a segment of allocortical hippocampus and ectosplenial area 26. Above the splenium, the hippocampal rudiment (IGr) or areas LB2 and HF (von Economo and Koskinas, 1925) form a single layer of densely packed ganglion cells that are heavily NFP-ir (Fig. 11). Adjacent to the IGr is the subicular rudiment (Sub) or area HE of von Economo and Koskinas which has many fewer and more dispersed neurons. These two areas together form the fasciolate gyrus on the dorsal surface of the corpus callosum. Although the term “intralimbic” has been used for this small gyrus (Retzius, 1896), the concept of an intralimbic gyrus in this region requires that this cortex is in fact limbic cortex. Since cortex that is not directly involved in expressing internal states of emotion and in directly regulating relevant autonomic outflow is probably not limbic, it is likely that this gyrus in not “intralimbic.”
Ectosplenial Area 26. Area 26 of Brodmann (1909), area LF of von Economo and Koskinas (1925), and area es of Braak (1979a) has an architecture that is almost the inverse of that expressed by the IGr and subicular rudiment. Area 26 has a very dense layer of granular neurons most of which are NFP-ir and there is a dense plexus of NFP-ir fibers in this layer (Fig. 11). Deep to the granular layer there are few pyramidal neurons, most of which are NFP-ir. This can be stated because the sections used for the SMI32 immunoreaction were counterstained with thionin and there are no thionin stained neurons that are not also SMI32-positive. Braak (1979a) observed that this deep layer was comprised of only the multiform neurons of layer VI.
Granular Area 29. The organization of retrosplenial cortex presents a number of difficult problems. It undergoes a series of significant transitions and its structure must be interpreted in terms of differentiation trends. Also, it is convoluted around the splenium of the corpus callosum and interpreting its organization and relationships with parahippocampal structures requires a trained eye. Finally, as valuable as the Brodmann map has been to human brain research, Talairach and Tournoux (1988) misinterpreted the location of Brodmann’s retrosplenial cortex by transposing area 30 to a posterior parahippocampal location.
Although the general architecture of RSC can be identified in Nissl-stained preparations (Vogt et al., 1995, 1997, 2001), the details of cytological organization are more clearly visible in immunohistochemical preparations for NeuN and SMI32. Figure 11 shows a suprasplenial level of area 29. The unifying feature of its two subdivisions is the granular layer that in lateral area 29l is directly adjacent to layer I and in medial area 29m it is deep to a layer of medium-sized pyramidal neurons termed layer III. The granular layer is outlined in Figure 11 to emphasize its presence in areas 26 and 29 and it is referred to as layer III/IV in areas 26 and 29l because it is an intermediate stage of differentiation of layers III and IV on the ventral bank of the cingulate gyrus (CGv). The granular layer contains many medium-sized pyramids that are NFP-ir and the associated NFP-r dendritic plexus is prominent in Figure 11 (SMI32). Although this is also termed the granular layer, the neurons are not small and “granular” as they are in the monkey and discussed below. In area 29m the density and sizes of neurons is reduced in layer IV because many medium-sized neurons are separated from this layer into a layer III (Figs. 11, 12, 15, 17). Beneath the granular layer in area 29l are poorly differentiated layers V and VI, while in area 29m layer V differentiates into sublayers Va and Vb. Many of the largest pyramids in area 29 are NFP-ir and they are located mainly in the deeper part of layer V in area 29l and the deeper part of layer Va in area 29m. Finally, there are some NFP-ir neurons in layer VI throughout area 29, although the associated plexus in layer VI is much less dense than in layers IV and Va.
Ectosplenial Area 26. Area 26 of Brodmann (1909), area LF of von Economo and Koskinas (1925), and area es of Braak (1979a) has an architecture that is almost the inverse of that expressed by the IGr and subicular rudiment. Area 26 has a very dense layer of granular neurons most of which are NFP-ir and there is a dense plexus of NFP-ir fibers in this layer (Fig. 11). Deep to the granular layer there are few pyramidal neurons, most of which are NFP-ir. This can be stated because the sections used for the SMI32 immunoreaction were counterstained with thionin and there are no thionin stained neurons that are not also SMI32-positive. Braak (1979a) observed that this deep layer was comprised of only the multiform neurons of layer VI.
Granular Area 29. The organization of retrosplenial cortex presents a number of difficult problems. It undergoes a series of significant transitions and its structure must be interpreted in terms of differentiation trends. Also, it is convoluted around the splenium of the corpus callosum and interpreting its organization and relationships with parahippocampal structures requires a trained eye. Finally, as valuable as the Brodmann map has been to human brain research, Talairach and Tournoux (1988) misinterpreted the location of Brodmann’s retrosplenial cortex by transposing area 30 to a posterior parahippocampal location.
Although the general architecture of RSC can be identified in Nissl-stained preparations (Vogt et al., 1995, 1997, 2001), the details of cytological organization are more clearly visible in immunohistochemical preparations for NeuN and SMI32. Figure 11 shows a suprasplenial level of area 29. The unifying feature of its two subdivisions is the granular layer that in lateral area 29l is directly adjacent to layer I and in medial area 29m it is deep to a layer of medium-sized pyramidal neurons termed layer III. The granular layer is outlined in Figure 11 to emphasize its presence in areas 26 and 29 and it is referred to as layer III/IV in areas 26 and 29l because it is an intermediate stage of differentiation of layers III and IV on the ventral bank of the cingulate gyrus (CGv). The granular layer contains many medium-sized pyramids that are NFP-ir and the associated NFP-r dendritic plexus is prominent in Figure 11 (SMI32). Although this is also termed the granular layer, the neurons are not small and “granular” as they are in the monkey and discussed below. In area 29m the density and sizes of neurons is reduced in layer IV because many medium-sized neurons are separated from this layer into a layer III (Figs. 11, 12, 15, 17). Beneath the granular layer in area 29l are poorly differentiated layers V and VI, while in area 29m layer V differentiates into sublayers Va and Vb. Many of the largest pyramids in area 29 are NFP-ir and they are located mainly in the deeper part of layer V in area 29l and the deeper part of layer Va in area 29m. Finally, there are some NFP-ir neurons in layer VI throughout area 29, although the associated plexus in layer VI is much less dense than in layers IV and Va.
Figure 11. Coronal sections through the ventral bank of the posterior cingulate gyrus showing the retrosplenial cortices. The granular layer of areas 26 and 29 is outlined to emphasize the commonality of this layer to ectosplenial area 26 and granular retrosplenial area 29. Note that the overall density and sizes of neurons in this layer in area 29m is reduced because large neurons in layer III of area 29m separate from the granular layer, while in area 29l, such differentiation has not occurred. In area 26 of both preparations, there are almost no neurons below the granular layer; i.e., there is essentially no internal pyramidal layer. Each layer in area 30 is shown to the left of the photographs including a variable layer IV.
It may seem arbitrary to term the first neuronal layer adjacent to layer I in area 29m as layer III rather than layer II. This selection is not the result of a simple pia to white matter counting but, rather, it is based on the facts of neuron structure and the principles of cortical transition in the posterior cingulate region. It has been suggested that this is not layer II in the monkey because these are not lancet-shaped neurons similar to those of layer II in area 23a and a distinct layer II appears only in the medial part of area 30 (Vogt, 1976). This fact is demonstrated below (Fig. 12) and confirms and extends observations in monkey RSC in the following ways. First, most neurons in layer III of area 29m are NFP-ir, while almost none are NFP-ir in layer II of area 23a and medial area 30. Second, there is a reduction in the overall density of neurons and the NFP-ir dendritic plexus in the lateral part of area 30 that is adjacent to area 29m (Figs. 11, 12). Third, the size and density of heavily NFP-ir neurons in layer III of area 29m contrasts with those in layer IIIc of area 30 (Fig. 11). It might then be concluded that neurons in layer III are most likely associated with layer IIIab as proposed for the monkey (Vogt, 1976). Thus, the cytoarchitecture of monkey and human cortices, Golgi studies in monkey and immmunohistochemical studies in human all point to the fact that layer III of area 29 is similar to that of layer IIIab in neocortex rather than layers II, IIIc, or IV.
The parvicellular layer IV of area 29m not only contains small and medium-sized pyramidal neurons, it also contains a number of large and solitary pyramids. These neurons are NFP-ir and are a distinguishing feature of area 29m along its full extent to its termination on the rostral bank of the CML. An example of these pyramids is circled in NeuN and SMI32 sections in Figure 12. At his magnification, it is also clear that layer III is comprised of many medium and large pyramidal neuronss that are heavily NFP-ir. These neurons likely are the undifferentiated counterparts of pyramids of layers II and IIIab in adjacent areas 30 and 23a.
It may seem arbitrary to term the first neuronal layer adjacent to layer I in area 29m as layer III rather than layer II. This selection is not the result of a simple pia to white matter counting but, rather, it is based on the facts of neuron structure and the principles of cortical transition in the posterior cingulate region. It has been suggested that this is not layer II in the monkey because these are not lancet-shaped neurons similar to those of layer II in area 23a and a distinct layer II appears only in the medial part of area 30 (Vogt, 1976). This fact is demonstrated below (Fig. 12) and confirms and extends observations in monkey RSC in the following ways. First, most neurons in layer III of area 29m are NFP-ir, while almost none are NFP-ir in layer II of area 23a and medial area 30. Second, there is a reduction in the overall density of neurons and the NFP-ir dendritic plexus in the lateral part of area 30 that is adjacent to area 29m (Figs. 11, 12). Third, the size and density of heavily NFP-ir neurons in layer III of area 29m contrasts with those in layer IIIc of area 30 (Fig. 11). It might then be concluded that neurons in layer III are most likely associated with layer IIIab as proposed for the monkey (Vogt, 1976). Thus, the cytoarchitecture of monkey and human cortices, Golgi studies in monkey and immmunohistochemical studies in human all point to the fact that layer III of area 29 is similar to that of layer IIIab in neocortex rather than layers II, IIIc, or IV.
The parvicellular layer IV of area 29m not only contains small and medium-sized pyramidal neurons, it also contains a number of large and solitary pyramids. These neurons are NFP-ir and are a distinguishing feature of area 29m along its full extent to its termination on the rostral bank of the CML. An example of these pyramids is circled in NeuN and SMI32 sections in Figure 12. At his magnification, it is also clear that layer III is comprised of many medium and large pyramidal neuronss that are heavily NFP-ir. These neurons likely are the undifferentiated counterparts of pyramids of layers II and IIIab in adjacent areas 30 and 23a.
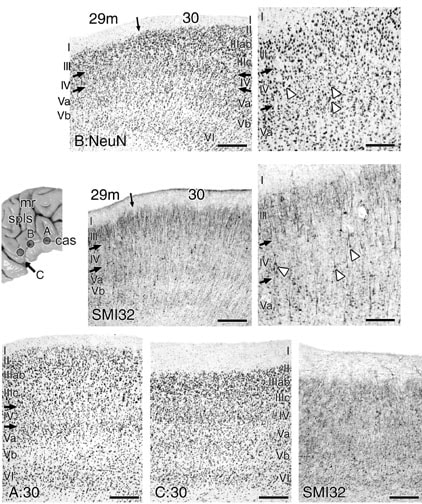
Figure 12. Three rostrocaudal levels of area 30 above the body of the corpus callosum (A), dorsal to the splenium (B), and caudal to the splenium and lateral to the CML as indicated with the arrow pointing below the CML (C). The medium-sized, NFP-ir neurons in the parvicellular layer IV are demonstrated and a few emphasized with white arrowheads. The level of the section at C is noted with brackets in Figure 15: NeuN. This level of area 30 is transitional to area 29m at its most caudal level; i.e., this is the terminal end of area 30. At this point, area 30 has a more densely granular layers II and IV and the sizes of layer III pyramids are reduced with less prominent NFP-ir dendrites.
Dysgranular Area 30. Although area 30 (Brodmann, 1909), LD (von Economo and Koskinas, 1925), Rsag (Rose, 1928), and rsm (Braak, 1979a) has been thoroughly analyzed histologically, the nature of its cytoarchitectural organization remains controversial. At each point of transition from allocortex to isocortex throughout the primate telencephalon, there is at least one area with the features of a dysgranular cortex. This is true for orbitofrontal cortex and the insula. In the insula, neurons in layer IV form islands as is characteristic of a layer that is of irregular thickness (Mufson et al., 1997). In orbitofrontal cortex, the intermediate area has a thin layer IV which can be difficult to detect where the layer III and V pyramids are particularly large (Hof et al., 1995). Since cortex on the CGv progresses through a number of transitions beginning with the allocortical indusium griseum and culminating in the isocortical area 23a, the presence of a dysgranular cortex in this region needs to be considered.
Brodmann (1909) referred to area 30 as agranular and von Economo and Koskinas (1925) were quite explicit that area LD is not just agranular but that the “granulous” layer of area LE (Brodmann’s area 29) is not continuous with the isocortical layer of area LC2 (Brodmann’s area 23). Although there is no doubt that layer III/IV in area 29 is not equivalent to layer IV in area 23a, von Economo (1929; Fig. 50) vacillated on the presence of a layer IV in LD and showed a layer III(IV) below layer III and the granular layer IV of area 29m is continuous with layer IV in area 30 and layer IV in area 23a in immunohistochemical preparations invalidating von Economo’s statement that they are not continuous.
The details of area 30 architecture are shown in Figure 12 for three rostrocaudal levels of this area. It has layers IIIab and IIIc which differentiate from layer III of area 29m. Although some small neurons are at the border between layers I and II, a layer II is most apparent at the medial edge of this area. Small neurons aggregate in layer IV and there are many instances where the layer is interrupted by bridges of neurons between layers IIIc and Va or where the small neurons interdigitate with those in layers IIIc and/or Va. The variability in layer IV thickness can be appreciated in Figure 12 where one level has a variable layer IV (B: NeuN), one has a thin but continuous layer (A: 30), and at the caudal level (C: 30) the granular neurons are either in a separate layer or intermingled with those in layer Va.
Area 30 has another feature that makes it stand out from all other areas in cingulate cortex. It has the highest relative density of NFP-ir neurons in layer IIIab when compared to other layers. Although some large neurons in layers IIIc and Va are NFP-ir, they are infrequent when compared to area 23a (Fig. 12B: SMI32). Although area 23b may have a similar number of NFP-ir neurons in layer IIIab, this area has the second highest density of NFP-ir neurons in layer IIIc (second only to area 31) and this contrasts strongly with the organization in area 30 where few of the layer IIIc neurons are NFP-ir. Finally, at caudal levels of area 30 (Fig. 12C: 30), the cortex is somewhat thinner and there is a more homogeneous distribution of NFP-ir neurons.
Dysgranular Area 30. Although area 30 (Brodmann, 1909), LD (von Economo and Koskinas, 1925), Rsag (Rose, 1928), and rsm (Braak, 1979a) has been thoroughly analyzed histologically, the nature of its cytoarchitectural organization remains controversial. At each point of transition from allocortex to isocortex throughout the primate telencephalon, there is at least one area with the features of a dysgranular cortex. This is true for orbitofrontal cortex and the insula. In the insula, neurons in layer IV form islands as is characteristic of a layer that is of irregular thickness (Mufson et al., 1997). In orbitofrontal cortex, the intermediate area has a thin layer IV which can be difficult to detect where the layer III and V pyramids are particularly large (Hof et al., 1995). Since cortex on the CGv progresses through a number of transitions beginning with the allocortical indusium griseum and culminating in the isocortical area 23a, the presence of a dysgranular cortex in this region needs to be considered.
Brodmann (1909) referred to area 30 as agranular and von Economo and Koskinas (1925) were quite explicit that area LD is not just agranular but that the “granulous” layer of area LE (Brodmann’s area 29) is not continuous with the isocortical layer of area LC2 (Brodmann’s area 23). Although there is no doubt that layer III/IV in area 29 is not equivalent to layer IV in area 23a, von Economo (1929; Fig. 50) vacillated on the presence of a layer IV in LD and showed a layer III(IV) below layer III and the granular layer IV of area 29m is continuous with layer IV in area 30 and layer IV in area 23a in immunohistochemical preparations invalidating von Economo’s statement that they are not continuous.
The details of area 30 architecture are shown in Figure 12 for three rostrocaudal levels of this area. It has layers IIIab and IIIc which differentiate from layer III of area 29m. Although some small neurons are at the border between layers I and II, a layer II is most apparent at the medial edge of this area. Small neurons aggregate in layer IV and there are many instances where the layer is interrupted by bridges of neurons between layers IIIc and Va or where the small neurons interdigitate with those in layers IIIc and/or Va. The variability in layer IV thickness can be appreciated in Figure 12 where one level has a variable layer IV (B: NeuN), one has a thin but continuous layer (A: 30), and at the caudal level (C: 30) the granular neurons are either in a separate layer or intermingled with those in layer Va.
Area 30 has another feature that makes it stand out from all other areas in cingulate cortex. It has the highest relative density of NFP-ir neurons in layer IIIab when compared to other layers. Although some large neurons in layers IIIc and Va are NFP-ir, they are infrequent when compared to area 23a (Fig. 12B: SMI32). Although area 23b may have a similar number of NFP-ir neurons in layer IIIab, this area has the second highest density of NFP-ir neurons in layer IIIc (second only to area 31) and this contrasts strongly with the organization in area 30 where few of the layer IIIc neurons are NFP-ir. Finally, at caudal levels of area 30 (Fig. 12C: 30), the cortex is somewhat thinner and there is a more homogeneous distribution of NFP-ir neurons.
Posterior Cingulate Cortex
The first isocortical area encountered in the transition that begins with the indusium griseum is area 23a. At its rostral border with area 24a' layer IV is thin and there is an intermingling of small neurons with large pyramids in layer Va (Fig. 13A: NeuN). It should be recalled that area 24' does not have a layer IV but it does have small neurons intermingled in layer Va. This feature, therefore, is a transitional aspect of rostral area 23a architecture. Caudally in area 23a, layer IV becomes more pronounced and, although a layer Va is present, the number of large neurons in it is surprisingly low. One of the key differences between areas 23a and 23b is that the latter area has a significantly greater number of neurons in layer Va.
Figure 13. Three rostrocaudal levels of area 23a show the architectural variability dorsal to the corpus callosum and on the CML. The greatest expansion of layer IV is dorsal to the splenium (B) where there is also highest density of large, NFP-ir layer III pyramids. Rostral and caudal to B, layer IV is somewhat thinner. Level C was selected as an explicit test of the hypothesis that areas 29 and 30 comprise most of the CML. This area does not have the properties of either area 29 or 30.
The greatest number of NFP-ir neurons in area 23a are in layer IIIc which is in striking contrast to area 30 where most are in layer IIIab. A moderate number of large neurons in layer Va also express NFP-ir and there is a fine mesh of NFP-ir dendrites throughout deep layer V and layer VI. As is the case for area 30, at caudal levels of area 23a there is a greater overall dispersion of NFP-ir neurons and dendrites that also includes some in layers II and IIIab (Fig. 13C: SMI32).
The border between areas 23a and 23b is clearly marked by a significant increase in the number of layer Va neurons and the thickness of layer IV. In the color figure below, the architecture of area 23b is shown. It is interesting to notice that the cell density in mid-area 23b is associated mainly with layer Va. At higher magnification, the layers IV/Va are shown with an intermingling of granularity in layers IV and Va.
The greatest number of NFP-ir neurons in area 23a are in layer IIIc which is in striking contrast to area 30 where most are in layer IIIab. A moderate number of large neurons in layer Va also express NFP-ir and there is a fine mesh of NFP-ir dendrites throughout deep layer V and layer VI. As is the case for area 30, at caudal levels of area 23a there is a greater overall dispersion of NFP-ir neurons and dendrites that also includes some in layers II and IIIab (Fig. 13C: SMI32).
The border between areas 23a and 23b is clearly marked by a significant increase in the number of layer Va neurons and the thickness of layer IV. In the color figure below, the architecture of area 23b is shown. It is interesting to notice that the cell density in mid-area 23b is associated mainly with layer Va. At higher magnification, the layers IV/Va are shown with an intermingling of granularity in layers IV and Va.
As is the case for area 23a, the rostral part of area 23b at its junction with area 24' has a thinner layer IV and a well formed layer Va that does not contain many small neurons. One of the outstanding features of the dorsal and posterior cingulate areas is the very high density of NFP-ir neurons throughout layer III. There are so many of these cells that even layers II, IV, and VI have a high density of them as shown in Figure 13B: SMI32. At caudal levels of area 23b there is a reduction in the overall density of NFP-ir neurons (Fig. 13C: SMI32).
As noted for the Brodmann area centroid analysis, area 31 surrounds the splenial sulci (spls) and Brodmann’s intentions are clear about its composition between the primary branches of these sulci. Once its primary structure is known, it is possible to determine the limits of this area with immunohistochemical preparations. Area 31 has the thickest layer IV on the cingulate gyrus. This layer can be compared with the same one in areas 23a and 23b in Figure 15 in the NeuN preparation or as a negative image in the SMI32 section. Layer Va is also well developed and it has the highest density of NFP-ir neurons in the posterior cingulate region (Fig. 15: SMI32). Finally, area 31 has the highest density of large, NFP-ir neurons in layer III in the entire cingulate gyrus. Although these neurons are obvious even at low magnification in Figure 15: SMI32, a 3X magnification is also shown for this area. As shown in Figure 15 and below in the subregional, postsplenial flat map (Fig. 18), area 31 forms the superior and posterior border of cingulate cortex. It extends ventrally and caudally to area 23b along the posterior border of the caudomedial subregion.
As noted for the Brodmann area centroid analysis, area 31 surrounds the splenial sulci (spls) and Brodmann’s intentions are clear about its composition between the primary branches of these sulci. Once its primary structure is known, it is possible to determine the limits of this area with immunohistochemical preparations. Area 31 has the thickest layer IV on the cingulate gyrus. This layer can be compared with the same one in areas 23a and 23b in Figure 15 in the NeuN preparation or as a negative image in the SMI32 section. Layer Va is also well developed and it has the highest density of NFP-ir neurons in the posterior cingulate region (Fig. 15: SMI32). Finally, area 31 has the highest density of large, NFP-ir neurons in layer III in the entire cingulate gyrus. Although these neurons are obvious even at low magnification in Figure 15: SMI32, a 3X magnification is also shown for this area. As shown in Figure 15 and below in the subregional, postsplenial flat map (Fig. 18), area 31 forms the superior and posterior border of cingulate cortex. It extends ventrally and caudally to area 23b along the posterior border of the caudomedial subregion.
Figure 15. Microphotographs of immunohistochemical preparations through the postsplenial posterior cingulate and retrosplenial regions and the junction with the PHG. The reconstruction paradox of RSC is clear because RSC is in the fundus of the CaS lateral to the CML not on the surface of the CML. The isocortical nature of areas 23a and 23b with their layer IV is apparent in both preparations. As is true for most cingulate areas, layer Va is prominent even in isocortex as noted. This case also demonstrates the clarity with which each area of CML can be assessed in standard coronal sections. Although area 29m does not appear on the CML in this case, its border with parahippocampal area 36'd on the PHGt is demonstrated. Area 31 has the thickest layer IV of any cingulate area and it has the highest level of NFP-ir in layer III that emphasizes its border with area 23b. The strip of area 31 cortex is a 3X magnification of the level in A: SMI32 marked with an asterisk and an arrow. The line with a double asterisk in A: SMI32 shows the level below which sections were enlarged for Figure 17.
Caudomedial Subregion
Brodmann (1909) showed areas 26, 29, and 30 extending around the full extent of the splenium of the corpus callosum, while von Economo (1929; his Fig. 58) provided a very careful reconstruction of this region and showed that the retrosplenial areas terminated posterior to the splenium at a level just caudal to the ventral edge of the splenium rather than fully embracing it per se. Although there is no doubt about the von Economo conclusions because of his carefully drawn map of the region, the same cannot be said of the Brodmann map. Brodmann’s map was reconstructed on the convoluted surface of the entire brain and must have made compromises to show the topology of each area in sulci and on gyral surfaces. We evaluated these issues and concluded that the RSC in Brodmann’s map does not extend far enough anteriorally, it does not extend onto the surface of the CML/CMSR, nor does it extend to the most ventral aspect of the splenium (Vogt et al., 2001). Each of these issues has a significant impact on how functional imaging studies localize activation sites in this region. Indeed, the use of Brodmann’s localizations without histological guidance led Talairach and Tournoux (1988) to mislocate the retrosplenial area 30 on the CML/CMSR. In light of the issues raised in structural and functional imaging studies and the reciprocal connections between perigenual ACC and the CMSR (Fig. 5 and associated text), the architecture of this ventral part of the posterior cingulate gyrus is considered in detail.
The PCC and CMSR have variable surface features as detailed in Figure 2 in “Introduction & Surface Morphology.” In the monkey there is a consistent pouch of cortex in the CMSR termed the caudomedial lobule (CML; Goldman-Rakic et al., 1984) and its surface is comprised mainly of areas 23a and 23b, while the rostral bank contains RSC (Vogt et al., 1987, 1997; Vogt, 1993). Although a similar conclusion has been reached in human brain (Vogt et al., 1995), the multiple gross morphologies of this cortex need consideration as do the details of transition to parahippocampal cortex. In instances in the human where a clearly defined CML is not present, the more general term caudomedial subregion (CMSR) is used. An overview of the posterior cingulate gyrus is provided in Figure 15 and the problem of reconstructing RSC on the convoluted surface of the human cortex is apparent therein. These sections (Fig. 15) show that area 23 directly overlies the ectosplenial and retrosplenial areas in the cas. The isocortical layer IV of areas 23a and 23b can be seen in cortex on the surface of the CML, while areas 29 and 30 underlie this cortex in the depths of the CaS and do not express a fully differentiated layer IV. It is not possible to accurately reconstruct these areas without flattening the cerebral cortex to show areas in the fundus of the CaS. At ventral levels the CA1 sector of the hippocampus replaces the indusium griseum (IGr) and area 29m terminates adjacent to the caudal parahippocampal areas 36'd and 36'v. Thus, it is impossible to accurately reconstruct this region on the convoluted surface because cortical folding doubles the extent of cortex around the splenium. This figure clarifies one of the important interpretations of Brodmann’s map in that his reconstruction reflects the ectosplenial and retrosplenial areas in the cas and not on the surface of the CML.
The PCC and CMSR have variable surface features as detailed in Figure 2 in “Introduction & Surface Morphology.” In the monkey there is a consistent pouch of cortex in the CMSR termed the caudomedial lobule (CML; Goldman-Rakic et al., 1984) and its surface is comprised mainly of areas 23a and 23b, while the rostral bank contains RSC (Vogt et al., 1987, 1997; Vogt, 1993). Although a similar conclusion has been reached in human brain (Vogt et al., 1995), the multiple gross morphologies of this cortex need consideration as do the details of transition to parahippocampal cortex. In instances in the human where a clearly defined CML is not present, the more general term caudomedial subregion (CMSR) is used. An overview of the posterior cingulate gyrus is provided in Figure 15 and the problem of reconstructing RSC on the convoluted surface of the human cortex is apparent therein. These sections (Fig. 15) show that area 23 directly overlies the ectosplenial and retrosplenial areas in the cas. The isocortical layer IV of areas 23a and 23b can be seen in cortex on the surface of the CML, while areas 29 and 30 underlie this cortex in the depths of the CaS and do not express a fully differentiated layer IV. It is not possible to accurately reconstruct these areas without flattening the cerebral cortex to show areas in the fundus of the CaS. At ventral levels the CA1 sector of the hippocampus replaces the indusium griseum (IGr) and area 29m terminates adjacent to the caudal parahippocampal areas 36'd and 36'v. Thus, it is impossible to accurately reconstruct this region on the convoluted surface because cortical folding doubles the extent of cortex around the splenium. This figure clarifies one of the important interpretations of Brodmann’s map in that his reconstruction reflects the ectosplenial and retrosplenial areas in the cas and not on the surface of the CML.
Figure 16. Area 30 dorsal to the splenium in the cas is compared with area 23a on the CML because no equivalent to area 30 has been identified in this latter region. The dysgranular nature of area 30 is emphasized at a point where layer IIIc and Va neurons intermingle (to the right of the double asterisks in A; NeuN). Area 30 also has a layer IV with many small and medium-sized NFP-ir neurons (SMI32). In contrast, area 23a has no neuron bridges over layer IV and only occasional solitary NFP-ir neurons in layer IV. Layer IIIc in area 23a has larger and more-dense NFP-ir neurons than is the case for area 30.
Although histological studies have not shown area 30 on the gyral surface in the CMSR, two essential issues are raised by the atlas interpretations of Brodmann’s map (Talairach and Tournoux, 1988). Does any gyral component of the CMSR/CML share cytoarchitectonic features with area 30 in the cas and how do the features of area 30 compare with those on the CML, including that area which most closely relates to area 30 cytoarchitectonics (i.e., area 23a which it borders)? In Figure 16, layer IV can be detected in areas 23 and 31. Since area 30 has a dysgranular layer IV that cannot be detected at the magnification of sections in Figure 15, it is unlikely that area 30 is part of the gyral surface at the isthmus. Direct comparison of a suprasplenial level of area 30 with cortex on the CML is provided in Figure 16 which has higher magnifications of layers IIIc-Va of area 30 (A. NeuN and SMI32) and photographs of area 23a on the CML which has the least differentiated layer IV in this region (i.e., between the brackets in Fig. 15: NeuN). The rationale for this comparison is that area 30 has such a poorly developed layer IV, one seeks to assess the likelihood that a similar area is on the CML. As noted earlier, area 30 has a layer IV which is interrupted by layer IIIc/Va neurons at the pair of asterisks in Figure 15 (NeuN). There are some medium-sized, NFP-ir pyramidal neurons throughout layer IV in area 30 and this reduces the clarity with which layer IV can be identified in the SMI32 preparations. In contrast, area 23a on the CML has a clear layer IV with only a few large and solitary layer IIIc or Va, NFP-ir neurons. There also are many more NFP-ir neurons in layer IIIc in area 23a than in area 30. As noted earlier, layer IIIc of area 30 has surprisingly few NFP-ir neurons in layer IIIc but the greatest relative density in layer IIIab. Finally, there are no neuronal bridges connecting layers IIIc and Va to the exclusion of a layer IV in area 23a, although layer IV in area 23a does have some solitary, NFP-ir neurons. Thus, area 30 is not expressed on the CML as suggested by hypotheses derived from Brodmann’s map.
Another major component of the CMSR is area 23b. This area has such a well-defined layer IV that it is easily observed at low magnifications as in Figure 15, which shows both layers IV and Va. These are the two most important features of area 23b; thickness and neuron densities in layers IV and Va. Many large pyramidal neurons in layer Va, most of which are NFP-ir, easily distinguish this area from area 23a and area 30. Finally, the distinctions between areas 23a and 23b are enhanced by the extensive size and number of NFP-ir neurons throughout layers IIIab and IIIc.
Although histological studies have not shown area 30 on the gyral surface in the CMSR, two essential issues are raised by the atlas interpretations of Brodmann’s map (Talairach and Tournoux, 1988). Does any gyral component of the CMSR/CML share cytoarchitectonic features with area 30 in the cas and how do the features of area 30 compare with those on the CML, including that area which most closely relates to area 30 cytoarchitectonics (i.e., area 23a which it borders)? In Figure 16, layer IV can be detected in areas 23 and 31. Since area 30 has a dysgranular layer IV that cannot be detected at the magnification of sections in Figure 15, it is unlikely that area 30 is part of the gyral surface at the isthmus. Direct comparison of a suprasplenial level of area 30 with cortex on the CML is provided in Figure 16 which has higher magnifications of layers IIIc-Va of area 30 (A. NeuN and SMI32) and photographs of area 23a on the CML which has the least differentiated layer IV in this region (i.e., between the brackets in Fig. 15: NeuN). The rationale for this comparison is that area 30 has such a poorly developed layer IV, one seeks to assess the likelihood that a similar area is on the CML. As noted earlier, area 30 has a layer IV which is interrupted by layer IIIc/Va neurons at the pair of asterisks in Figure 15 (NeuN). There are some medium-sized, NFP-ir pyramidal neurons throughout layer IV in area 30 and this reduces the clarity with which layer IV can be identified in the SMI32 preparations. In contrast, area 23a on the CML has a clear layer IV with only a few large and solitary layer IIIc or Va, NFP-ir neurons. There also are many more NFP-ir neurons in layer IIIc in area 23a than in area 30. As noted earlier, layer IIIc of area 30 has surprisingly few NFP-ir neurons in layer IIIc but the greatest relative density in layer IIIab. Finally, there are no neuronal bridges connecting layers IIIc and Va to the exclusion of a layer IV in area 23a, although layer IV in area 23a does have some solitary, NFP-ir neurons. Thus, area 30 is not expressed on the CML as suggested by hypotheses derived from Brodmann’s map.
Another major component of the CMSR is area 23b. This area has such a well-defined layer IV that it is easily observed at low magnifications as in Figure 15, which shows both layers IV and Va. These are the two most important features of area 23b; thickness and neuron densities in layers IV and Va. Many large pyramidal neurons in layer Va, most of which are NFP-ir, easily distinguish this area from area 23a and area 30. Finally, the distinctions between areas 23a and 23b are enhanced by the extensive size and number of NFP-ir neurons throughout layers IIIab and IIIc.
Posterior Parahippocampal Areas
The development of the retrosplenial areas and their differentiation from parahippocampal areas has been elegantly shown with immunohistochemical analyses of coronal sections in the monkey by Berger et al. (1997). This latter study showed that in the CaS there is an arch of neurotensinergic neurons in the deep layer of areas 29 and 30 and the elaboration of these areas and their apposition to parahippocampal areas 27 and the parasubiculum. Although the retrosplenial and presubicular/parasubicular areas abut in the callosal sulcus just ventral to the splenium, in the human there is an intermediate level of transition between the posterior cingulate and more lateral parts of parahippocampal cortex. Ventral to posterior cingulate and retrosplenial cortices are two divisions of posterior parahippocampal cortex that share features with area 36. Area 36 has well defined layers II and Va and a dense layer VI. Layer III is broad and the pyramidal neurons are relatively uniform in size (Vogt et al., 2001). The division of layer III into a superficial layer IIIab and deeper layer IIIc is possible, although the neurons in layer IIIc do not reach the size and dispersion characteristic of the cingulate neocortical areas.
In contrast to area 36, area 36'd has a less differentiated layer III (i.e., no ab/c divisions) and differentiation with layer II is less apparent. Layer Va is present but not at the clarity seen in area 36. Area 36'v is more homogeneous overall with more differentiation in layer II and a more homogeneous distribution of neurons in layers III-VI (Vogt et al., 2001). Area 36'd has a dense plexus of dendrites and neurons in layer III and a smaller one in layer Va, although this is broader than its equivalent in areas 23a and 23b. Area 36'v, in contrast, has only a narrow plexus of SMI32-ir dendrites and neurons in layer III, while that in layer V is much broader than in area 36'd. In the case shown in Figure 17, there was no instance in which area 29m extended onto the surface of the CML. he transitional zone of parahippocampal cortex reached from the postsplenial dimple (at the border between areas 23a and 36'd) to the ventral edge of the transitional PHG.
In contrast to area 36, area 36'd has a less differentiated layer III (i.e., no ab/c divisions) and differentiation with layer II is less apparent. Layer Va is present but not at the clarity seen in area 36. Area 36'v is more homogeneous overall with more differentiation in layer II and a more homogeneous distribution of neurons in layers III-VI (Vogt et al., 2001). Area 36'd has a dense plexus of dendrites and neurons in layer III and a smaller one in layer Va, although this is broader than its equivalent in areas 23a and 23b. Area 36'v, in contrast, has only a narrow plexus of SMI32-ir dendrites and neurons in layer III, while that in layer V is much broader than in area 36'd. In the case shown in Figure 17, there was no instance in which area 29m extended onto the surface of the CML. he transitional zone of parahippocampal cortex reached from the postsplenial dimple (at the border between areas 23a and 36'd) to the ventral edge of the transitional PHG.
Figure 17. Areas at the transition of the posterior cingulate and retrosplenial areas to parahippocampal cortex in coronal section. These sections are magnified from the level noted with double asterisks in Figure 24. Area 23a merges with area 36'd and area 29m merges with area 36'v. Since the arrow between areas 23a and 36'd is also at the terminal end of the PSD, the ventral parts of these sections is the transitional part of the PHG. The pullout of area 29m is a 2X magnification to emphasize the cytological features of transition from area 29m to area 36'v.
The relative density of neurons in layer IV of area 29m increases around the splenium and to its termination either in the ventral part of the CaS or the rostral lip of the CML/CMSR. Termination in the cas is shown in the pullout in Figure 17 at a level that begins at the double asterisk in Figure 15. The transition at this point is with area 36'v. The NFP-ir plexus in layers III and IV are apparent and disappear as area 29m merges with area 36'v. Area 36'v only has a NFP-ir plexus in layer V. Features of area 36'v that distinguish it from area 29m include a broad layers II-III composed of more uniform, medium-sized pyramidal neurons, a poorly defined layer IV, and an infragranular layer dominated by a uniform layer V and a narrow layer VI.
The relative density of neurons in layer IV of area 29m increases around the splenium and to its termination either in the ventral part of the CaS or the rostral lip of the CML/CMSR. Termination in the cas is shown in the pullout in Figure 17 at a level that begins at the double asterisk in Figure 15. The transition at this point is with area 36'v. The NFP-ir plexus in layers III and IV are apparent and disappear as area 29m merges with area 36'v. Area 36'v only has a NFP-ir plexus in layer V. Features of area 36'v that distinguish it from area 29m include a broad layers II-III composed of more uniform, medium-sized pyramidal neurons, a poorly defined layer IV, and an infragranular layer dominated by a uniform layer V and a narrow layer VI.
Subregional Postsplenial Flat Map
Cytoarchitectural studies of human cortex require flat map reconstruction of the areas identified because 50% or more of the cortical surface is in the sulci and this is particularly true for the posterior cingulate region. One of the problems interpreting Brodmann’s map (1909) is his use of the convoluted medial surface rather than an unfolded counterpart. A flat map of the entire cingulate gyrus is available in which the fundus of the CaS is represented and areas 29 and 30 are plotted on the CGv (Vogt et al., 1995). As is true for flat maps of the entire brain, however, flat maps of the entire cingulate cortex result in significant distortions in all areas due to the need to warp all areas to fit a two dimensional space and still retain basic gross morphological relationships. Subregional flat maps alleviate this problem because they are limited to a subsector of cingulate cortex, flattening is performed in only one direction rather than two, and it is not influenced by flattening in other parts of cingulate cortex to form a full map. von Economo (1929; Fig. 58) provided the first posterior cingulate subregional map, although it was not flattened.
Figure 18. Subregional postsplenial flat map including the entire CML that is a linear expansion of posterior cingulate, retrosplenial, and parahippocampal cortices in a single direction (large arrow on the scale bar, caudal from the fundus of the CaS). The dorsal asterisk on the right extends from the depths of the cas to an equivalent starting point on the flat map on the left and the same is true for the rostral edge of the PHG/PHGt. Areas on the CGv are enclosed by thick lines on both sides of the callosal sulcus, while those on the gyral surface are extended to the right on the CML.
Figure 18 shows a case in which postsplenial cortex was sectioned in the plane of the flat map reconstruction to assure precision in relating features on the map to histological observations. Asterisks on the convoluted surface on the left are used to orient the flat map at the right. The first one is at the cas and emphasizes that flattening was started at the fundus of the cas, while the second asterisk shows the rostral edge of the transitional PHG in both drawings. The depths of the cas was determined according to the curvature of the splenium and each area represented according to the length of a line drawn through each area at a midcortical level (i.e., usually between layers IV and V). Heavy lines in Figure 18 represent cortex on the CGv, i.e., cortex in the CaS, and the caudal edge of the CML, while in the ventral part of the map they represent the rostral and caudal limits of the PHGt and PHG. The direction and calibration of flattening in this case is shown with the arrow and scale bar, respectively.
This map shows that almost all of RSC is on the CGv in the cas. Area 30 ends just dorsal the area 29m and this case has a small termination of area 30 on the rostral edge of the CML. Because standardized atlases based on Brodmann’s map show that most of the CMR is comprised of area 30, it is of interest to determine the actual extent of area 30 on the CML in this case. Since the map was produced by flattening in only one dimension rather than two dimensions, it accurately represents the relative surface area for each component of the CML and cortex on the CGv, proportions of area occupied by each cortical area was calculated directly from the flat map. Area 30 comprises 0.8% of CML (1.63 mm2 ÷ 200 mm2), while it is 33% of area on the CGv at the level of the CML (31 mm2 ÷ 94 mm2). In contrast, area 29m comprises 2.8% of the rostral edge of the CML, while it is 26% of cortex on the CGv. Thus, both areas 30 and 29m are almost entirely located within the CaS and all of areas 29l and 26 are in this sulcus. Finally, the PHGt contains a small rostral extension of area 29m and areas 36'd and 36'v and neither area 29m or area 30 appeared on the CML. Thus, area 30 does not comprise a substantive part of the CML.
Figure 18 shows a case in which postsplenial cortex was sectioned in the plane of the flat map reconstruction to assure precision in relating features on the map to histological observations. Asterisks on the convoluted surface on the left are used to orient the flat map at the right. The first one is at the cas and emphasizes that flattening was started at the fundus of the cas, while the second asterisk shows the rostral edge of the transitional PHG in both drawings. The depths of the cas was determined according to the curvature of the splenium and each area represented according to the length of a line drawn through each area at a midcortical level (i.e., usually between layers IV and V). Heavy lines in Figure 18 represent cortex on the CGv, i.e., cortex in the CaS, and the caudal edge of the CML, while in the ventral part of the map they represent the rostral and caudal limits of the PHGt and PHG. The direction and calibration of flattening in this case is shown with the arrow and scale bar, respectively.
This map shows that almost all of RSC is on the CGv in the cas. Area 30 ends just dorsal the area 29m and this case has a small termination of area 30 on the rostral edge of the CML. Because standardized atlases based on Brodmann’s map show that most of the CMR is comprised of area 30, it is of interest to determine the actual extent of area 30 on the CML in this case. Since the map was produced by flattening in only one dimension rather than two dimensions, it accurately represents the relative surface area for each component of the CML and cortex on the CGv, proportions of area occupied by each cortical area was calculated directly from the flat map. Area 30 comprises 0.8% of CML (1.63 mm2 ÷ 200 mm2), while it is 33% of area on the CGv at the level of the CML (31 mm2 ÷ 94 mm2). In contrast, area 29m comprises 2.8% of the rostral edge of the CML, while it is 26% of cortex on the CGv. Thus, both areas 30 and 29m are almost entirely located within the CaS and all of areas 29l and 26 are in this sulcus. Finally, the PHGt contains a small rostral extension of area 29m and areas 36'd and 36'v and neither area 29m or area 30 appeared on the CML. Thus, area 30 does not comprise a substantive part of the CML.
References
Bench, C. J., Frith, C. D., Grasby, P. M., Friston, K. J., Paulesu, E., Frackowiak, R. S. J., and Dolan, R. J. (1992). Patterns of cerebral activation during the Stroop colour word interference task: a positron emission tomography study. Neuropsychologia 31, 907-922.
Berger, B., Alvarez, C., and Pelaprat, D. (1997). Retrosplenial/presubicular continuum in primates: a developmental approach in fetal macaques using neurotensin and parvalbumin as markers. Devel. Brain Res. 101, 207-224.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109, 219-233.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. (1979b). Pigment architecture of the human telencephalic cortex. V. Regio anterogenualis. Cell Tissue Res. 204, 441-451.
Brodmann, K. (1909). "Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues." Barth, Leipzig.
Dum, R. P. and Strick, P. L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667-689.
Dum, R. P. and Strick, P. L. (1993). Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 415-441. Birkhäuser, Boston.
von Economo, C. and Koskinas, G. N. (1925). "Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen." Springer, Berlin.
von Economo, C. (1929). "The Cytoarchitectonics of the Human Cerebral Cortex." Oxford University Press, London.
Hof, P. R., Mufson, E. J., and Morrison, J. H. (1995). Human orbitofrontal cortex cytoarchitecture and quantitative immunohistochemical parcellation. J. Comp. Neurol. 359, 48-68.
Luppino, G., Matelli, M., Camarda, R. M., Gallese, V., and Rizzolatti, G. (1991). Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J. Comp. Neurol. 311, 463-482.
Morecraft, R. J. and Van Hoesen, G. W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in Areas 24c and 23c. J. Comp. Neurol. 322, 471-489.
Morecraft, R. J. and Van Hoesen, G. W. (1998). Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res. Bull. 45, 209-232.
Mufson, E. J., Sobreviela, T., and Kordoer, J. H. (1997). Chemical neuroanatomy of the primate insula cortex: relationship to cytoarchitectonics, connectivity function, and neurodegeneration. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, Elsevier, Amsterdam.
Mullen, R. J., Buck, C. R., and Smith, A. M. (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201-211.
Nimchinsky, E. A., Vogt, B. A., Morrison, J. H., and Hof, P. R. (1995). Spindle neurons of the human anterior cingulate cortex. J. Comp. Neurol. 355, 27-37.
Nimchinsky, E. A., Hof, P. R., Young, W. G., and Morrison, J. H. (1996). Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate motor areas of the macaque monkey. J. Comp. Neurol. 374, 136-160.
Nimchinsky, E. A., Vogt, B. A., Morrison, J. H., and Hof, P. R. (1997). Neurofilament and calcium-binding proteins in the human cingulate cortex. J. Comp. Neurol. 384, 597-620.
Nimchinsky, E.A., Gilissen, E., Allman, J.M., Perl, D.P., Erwin, J.M., Hof, P.R. (1999) A neuronal morphologic type unique to human and great apes. Proc. Natl. Acad. Sci. 96, 5268-5273.
Pardo, J. V., Pardo, P. J., Janer, K. W., and Raichle, M. E. (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci.USA 87, 256-259.
Raichle, M. E., Fiez, J. A., Videen, T. O., MacLeod, A.-M., Pardo, J. V., Fox, P. T., and Petersen, S. E. (1994). Practice-related changes in Alzheimer's disease. Cereb. Cortex 4, 8-26.
Rizzolatti, G., Luppino, G., and Matelli, M. (1996). The classic supplementary motor area ids formed by two independent areas. In "Advances in Neurology" (H. O. Lüders, Ed.), Vol. 70: Supplementary Sensorimotor Areas, pp. 45-56. Lippincott-Raven Publishers, Philadelphia.
Rose, M. (1928). Gyrus limbicus anterior and Regio retrosplenialis (Cortex holoprotoptychos quinquestratificatus). Vergleichende Architektonik bei Tier und Mensch. J. Psychol. Neurol. 35, 65-173.
Sarkisov, S. A., Filimonoff, I. N., Kononowa, E. P., Preobraschenskaja, I. S., and Kukuew, E. A. (1955). "Atlas of the cytoarchitectonics of the human cerebral cortex." Medgiz, Moscow.
Schlaug, G., Armstrong, E., Schleicher, A., and Zilles, K. (1993). Layer V pyramidal cells in the adult human cingulate cortex. Anat .Embryol. 187, 515-522.
Talairach, J. and Tournoux, P. (1988). "Co-Planar Stereotaxic Atlas of the Human Brain." Thieme Medical Publishers, New York.
Van Hoesen, G. W., Morecraft, R. J., and Vogt, B. A. (1993). Connections of the monkey cingulate cortex. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 249-284. Birkhäuser, Boston.
Vogt, B. A. (1976). Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. J. Comp. Neurol. 169, 63-98.
Vogt, B. A. and Peters, A. (1981). Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J. Comp. Neurol. 195, 603-625.
Vogt, B. A., Pandya, D. N., and Rosene, D. L. (1987). Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J. Comp. Neurol. 262, 256-270.
Vogt, B. A. and Barbas, H. (1988). Structure and connections of the cingulate vocalization region in the rhesus monkey. In "The Physiological Control of Mammalian Vocalization" (J. D. Newman, Ed.), pp. 203-225. Plenum, New York.
Vogt, B. A. (1993). Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 19-70. Birkhäuser, Boston.
Vogt, B. A., Nimchinsky, E. A., Vogt, L. J., and Hof, P. R. (1995). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Vogt, B. A., Vogt, L. J., Nimchinsky, E. A., and Hof, P. R. (1997). Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer's disease. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, pp. 455-528. Elsevier, Amsterdam.
Vogt, B. A., Vogt, L. J., Perl, D. P., and Hof, P. R. (2001). Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J. Comp. Neurol .
Bench, C. J., Frith, C. D., Grasby, P. M., Friston, K. J., Paulesu, E., Frackowiak, R. S. J., and Dolan, R. J. (1992). Patterns of cerebral activation during the Stroop colour word interference task: a positron emission tomography study. Neuropsychologia 31, 907-922.
Berger, B., Alvarez, C., and Pelaprat, D. (1997). Retrosplenial/presubicular continuum in primates: a developmental approach in fetal macaques using neurotensin and parvalbumin as markers. Devel. Brain Res. 101, 207-224.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109, 219-233.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. (1979b). Pigment architecture of the human telencephalic cortex. V. Regio anterogenualis. Cell Tissue Res. 204, 441-451.
Brodmann, K. (1909). "Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues." Barth, Leipzig.
Dum, R. P. and Strick, P. L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667-689.
Dum, R. P. and Strick, P. L. (1993). Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 415-441. Birkhäuser, Boston.
von Economo, C. and Koskinas, G. N. (1925). "Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen." Springer, Berlin.
von Economo, C. (1929). "The Cytoarchitectonics of the Human Cerebral Cortex." Oxford University Press, London.
Hof, P. R., Mufson, E. J., and Morrison, J. H. (1995). Human orbitofrontal cortex cytoarchitecture and quantitative immunohistochemical parcellation. J. Comp. Neurol. 359, 48-68.
Luppino, G., Matelli, M., Camarda, R. M., Gallese, V., and Rizzolatti, G. (1991). Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: An intracortical microstimulation study in the macaque monkey. J. Comp. Neurol. 311, 463-482.
Morecraft, R. J. and Van Hoesen, G. W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in Areas 24c and 23c. J. Comp. Neurol. 322, 471-489.
Morecraft, R. J. and Van Hoesen, G. W. (1998). Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res. Bull. 45, 209-232.
Mufson, E. J., Sobreviela, T., and Kordoer, J. H. (1997). Chemical neuroanatomy of the primate insula cortex: relationship to cytoarchitectonics, connectivity function, and neurodegeneration. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, Elsevier, Amsterdam.
Mullen, R. J., Buck, C. R., and Smith, A. M. (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201-211.
Nimchinsky, E. A., Vogt, B. A., Morrison, J. H., and Hof, P. R. (1995). Spindle neurons of the human anterior cingulate cortex. J. Comp. Neurol. 355, 27-37.
Nimchinsky, E. A., Hof, P. R., Young, W. G., and Morrison, J. H. (1996). Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate motor areas of the macaque monkey. J. Comp. Neurol. 374, 136-160.
Nimchinsky, E. A., Vogt, B. A., Morrison, J. H., and Hof, P. R. (1997). Neurofilament and calcium-binding proteins in the human cingulate cortex. J. Comp. Neurol. 384, 597-620.
Nimchinsky, E.A., Gilissen, E., Allman, J.M., Perl, D.P., Erwin, J.M., Hof, P.R. (1999) A neuronal morphologic type unique to human and great apes. Proc. Natl. Acad. Sci. 96, 5268-5273.
Pardo, J. V., Pardo, P. J., Janer, K. W., and Raichle, M. E. (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc. Natl. Acad. Sci.USA 87, 256-259.
Raichle, M. E., Fiez, J. A., Videen, T. O., MacLeod, A.-M., Pardo, J. V., Fox, P. T., and Petersen, S. E. (1994). Practice-related changes in Alzheimer's disease. Cereb. Cortex 4, 8-26.
Rizzolatti, G., Luppino, G., and Matelli, M. (1996). The classic supplementary motor area ids formed by two independent areas. In "Advances in Neurology" (H. O. Lüders, Ed.), Vol. 70: Supplementary Sensorimotor Areas, pp. 45-56. Lippincott-Raven Publishers, Philadelphia.
Rose, M. (1928). Gyrus limbicus anterior and Regio retrosplenialis (Cortex holoprotoptychos quinquestratificatus). Vergleichende Architektonik bei Tier und Mensch. J. Psychol. Neurol. 35, 65-173.
Sarkisov, S. A., Filimonoff, I. N., Kononowa, E. P., Preobraschenskaja, I. S., and Kukuew, E. A. (1955). "Atlas of the cytoarchitectonics of the human cerebral cortex." Medgiz, Moscow.
Schlaug, G., Armstrong, E., Schleicher, A., and Zilles, K. (1993). Layer V pyramidal cells in the adult human cingulate cortex. Anat .Embryol. 187, 515-522.
Talairach, J. and Tournoux, P. (1988). "Co-Planar Stereotaxic Atlas of the Human Brain." Thieme Medical Publishers, New York.
Van Hoesen, G. W., Morecraft, R. J., and Vogt, B. A. (1993). Connections of the monkey cingulate cortex. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 249-284. Birkhäuser, Boston.
Vogt, B. A. (1976). Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. J. Comp. Neurol. 169, 63-98.
Vogt, B. A. and Peters, A. (1981). Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J. Comp. Neurol. 195, 603-625.
Vogt, B. A., Pandya, D. N., and Rosene, D. L. (1987). Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J. Comp. Neurol. 262, 256-270.
Vogt, B. A. and Barbas, H. (1988). Structure and connections of the cingulate vocalization region in the rhesus monkey. In "The Physiological Control of Mammalian Vocalization" (J. D. Newman, Ed.), pp. 203-225. Plenum, New York.
Vogt, B. A. (1993). Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 19-70. Birkhäuser, Boston.
Vogt, B. A., Nimchinsky, E. A., Vogt, L. J., and Hof, P. R. (1995). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Vogt, B. A., Vogt, L. J., Nimchinsky, E. A., and Hof, P. R. (1997). Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer's disease. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, pp. 455-528. Elsevier, Amsterdam.
Vogt, B. A., Vogt, L. J., Perl, D. P., and Hof, P. R. (2001). Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J. Comp. Neurol .