Cingulate Gyrus: Introduction and Surface Morphology
The cingulate gyrus is the most prominent cortical feature on the medial surface of the human brain. It extends from the lamina terminalis rostral to the anterior commissure, around the genu of the corpus callosum, over the body of the callosum, and just ventral to the splenium. The cingulate gyrus forms the dorsal component of the grand limbic lobe of Broca (1878) and has a major role in most theories of emotion (MacLean, 1993). Although it forms a single and continuous structure, the cingulate gyrus is structurally and functionally heterogeneous. Efforts to identify a structural motif that is responsible for the role of cingulate cortex in emotion and “a” limbic system have failed as have efforts to characterize a single cingulate function such as attention. Because assessing the structure of human cingulate cortex may uncover organizational principles that pertain to the function of the entire brain, characterizing the structural heterogeneity of human cingulate cortex has been one of the major challenges of modern neuroscience.
The cingulum bundle is a white matter tract that underlies cingulate cortex and all connections entering and exiting the cingulate gyrus pass through this bundle. In addition, there are axons of passage that do not arise in cingulate cortex nor do they terminate there. These pathways include projections between prefrontal and parahippocampal cortices (Nauta, 1964; Goldman-Rakic et al., 1984) and projections to the median raphe nucleus that terminate in the dorsal hippocampus (Azmitia and Segal, 1978). The cingulum bundle has been the target of neurosurgical procedures for alleviation of chronic pain and psychiatric disorders including anxiety, major depression and obsessive-compulsive disorder. When the primary target of the procedure is the cingulum bundle, the procedure is termed a cingulumotomy and when the target is cingulate cortex itself and damage to the cingulum bundle is secondary to the procedure, the ablative technique is termed cingulotomy. Finally, the term cingulum was used in the early part of the last millennium when Roman soldiers and clerics wore a belt or sash and woman wore an undergarment for breast support termed a cingulum. In the context of modern neuroanatomy, the cingulum bundle is the longitudinally oriented aggregate of myelinated axons that underlies cingulate cortex and looks like a belt or sash surrounding the corpus callosum when the overlying gray matter has been removed.
In decades past, the cingulate gyrus was viewed as a relatively simple region with anterior and posterior divisions and studies often assumed that Brodmann’s (1909) maps of the medial surface were essentially correct. Recent macaque monkey connection studies, human functional imaging, and the cytoarchitectural work of Braak (1976, 1979a, b) have shown that the primate cingulate gyrus is more complex than originally envisioned by Brodmann and major revisions are necessary. In most instances the Brodmann areas are not cytologically uniform and do not perform a single function. We begin this presentation with a consideration of the variations in the surface morphology of the cingulate gyrus and comments on brain maps that had a significant influence on current views of cingulate cytoarchitecture. Brodmann area “centroids” are identified and histological features of each area shown in Nissl preparations to accommodate the lack of a full documentation supporting his maps. Much of text defines the cytological criteria for each area based on new human cases prepared with immunohistochemical techniques not previously applied to the entire human cingulate gyrus. The cytological criteria for each area in the human cingulate gyrus are now available as are the patterns of transition that lead from adjacent allocortical areas to fully developed isocortex. The heterogeneity of cingulate structure and function that baffled neuroscientists for generations is close to resolution and this will in turn provide a clear path to elucidating mechanisms of disease and their therapeutic amelioration through study of cingulate cortex.
A neurobiological model of cingulate organization incorporating key structure/function correlations requires the full armamentarium of neuroscience because no single methodology or theoretical framework has proven adequate to model this complex region. Indeed, one of the jewels of cingulate neurobiology is being produced by imaging human cerebral activation under carefully controlled neuropsychological testing conditions. Even this body of work, however, cannot stand in a vacuum and must become part of a larger structure/function model. The first such model sought to localize key functions within a tripartite morphology of monkey cortex; anterior cingulate, posterior cingulate and retrosplenial cortices (Vogt et al., 1992). In subsequent years that included a spate of human imaging studies, this model was extended to the current four-region model (Vogt et al., 1997). These evolving models of medial cortical structure and function seek to identify the unique contributions of each of the 20 cingulate areas to particular functions. In the long run, such studies will yield a wealth of information about the mechanisms of neurodegeneration in neurologic and psychiatric diseases and mechanisms of action of new drugs, neurosurgical outcomes, and for modulating consciousness in alternative mind/body methods.
The cingulum bundle is a white matter tract that underlies cingulate cortex and all connections entering and exiting the cingulate gyrus pass through this bundle. In addition, there are axons of passage that do not arise in cingulate cortex nor do they terminate there. These pathways include projections between prefrontal and parahippocampal cortices (Nauta, 1964; Goldman-Rakic et al., 1984) and projections to the median raphe nucleus that terminate in the dorsal hippocampus (Azmitia and Segal, 1978). The cingulum bundle has been the target of neurosurgical procedures for alleviation of chronic pain and psychiatric disorders including anxiety, major depression and obsessive-compulsive disorder. When the primary target of the procedure is the cingulum bundle, the procedure is termed a cingulumotomy and when the target is cingulate cortex itself and damage to the cingulum bundle is secondary to the procedure, the ablative technique is termed cingulotomy. Finally, the term cingulum was used in the early part of the last millennium when Roman soldiers and clerics wore a belt or sash and woman wore an undergarment for breast support termed a cingulum. In the context of modern neuroanatomy, the cingulum bundle is the longitudinally oriented aggregate of myelinated axons that underlies cingulate cortex and looks like a belt or sash surrounding the corpus callosum when the overlying gray matter has been removed.
In decades past, the cingulate gyrus was viewed as a relatively simple region with anterior and posterior divisions and studies often assumed that Brodmann’s (1909) maps of the medial surface were essentially correct. Recent macaque monkey connection studies, human functional imaging, and the cytoarchitectural work of Braak (1976, 1979a, b) have shown that the primate cingulate gyrus is more complex than originally envisioned by Brodmann and major revisions are necessary. In most instances the Brodmann areas are not cytologically uniform and do not perform a single function. We begin this presentation with a consideration of the variations in the surface morphology of the cingulate gyrus and comments on brain maps that had a significant influence on current views of cingulate cytoarchitecture. Brodmann area “centroids” are identified and histological features of each area shown in Nissl preparations to accommodate the lack of a full documentation supporting his maps. Much of text defines the cytological criteria for each area based on new human cases prepared with immunohistochemical techniques not previously applied to the entire human cingulate gyrus. The cytological criteria for each area in the human cingulate gyrus are now available as are the patterns of transition that lead from adjacent allocortical areas to fully developed isocortex. The heterogeneity of cingulate structure and function that baffled neuroscientists for generations is close to resolution and this will in turn provide a clear path to elucidating mechanisms of disease and their therapeutic amelioration through study of cingulate cortex.
A neurobiological model of cingulate organization incorporating key structure/function correlations requires the full armamentarium of neuroscience because no single methodology or theoretical framework has proven adequate to model this complex region. Indeed, one of the jewels of cingulate neurobiology is being produced by imaging human cerebral activation under carefully controlled neuropsychological testing conditions. Even this body of work, however, cannot stand in a vacuum and must become part of a larger structure/function model. The first such model sought to localize key functions within a tripartite morphology of monkey cortex; anterior cingulate, posterior cingulate and retrosplenial cortices (Vogt et al., 1992). In subsequent years that included a spate of human imaging studies, this model was extended to the current four-region model (Vogt et al., 1997). These evolving models of medial cortical structure and function seek to identify the unique contributions of each of the 20 cingulate areas to particular functions. In the long run, such studies will yield a wealth of information about the mechanisms of neurodegeneration in neurologic and psychiatric diseases and mechanisms of action of new drugs, neurosurgical outcomes, and for modulating consciousness in alternative mind/body methods.
Surface Morphology
The surface features of human cingulate cortex are quite variable and it is possible that each individual has their own “fingerprint” of medial surface features. To the extent that deep sulci are the most consistent, superficial or tertiary sulci may be excluded from surface feature classifications. Although Retzius (1896) performed the first systematic assessment of the surface cerebral morphology, the work of Ono et al. (1990) is more readily available and provides high quality macrophotographs of the 25 postmortem specimens in their analysis that includes the cingulate gyrus. Ono et al. (1990) report that the only large, main sulcus on the medial surface is the cingulate sulcus, which in usually not interrupted (58% of cases). The medial surface has a number of short, main sulci including the heavily branched splenial sulcus (spls) and the superior (srs) and inferior rostral (irs) sulci. Although Ono et al. use the term subparietal sulcus for the former sulcus, spls is used here because it is surrounded by dorsal and posterior cingulate area 31 and suggests an appropriate relation with the splenium. Parietal area 7m lies dorsal to area 31, thus, this sulcus is not subparietal but, rather, it is intraposterior cingulate. Although the single cingulate sulcus, with or without interruptions, is the most common form (76% on right or left hemispheres; Ono et al., 1990), there are other patterns. The other most frequent pattern is the double parallel pattern where the paracingulate sulcus (pcgs) forms a long path dorsal to the cingulate sulcus (cgs). Ono et al. observed this in 24% of either hemisphere. Figure 1 presents a number of examples of these various patterns observed in our postmortem series. These include a single cgs without (Fig. 1A) or with (Fig. 1B) segmentation and the double parallel cgs without (Fig. 1C) or with (Fig. 1D) segmentation. Finally, even when the pcgs is not robust, there is a superior cingulate gyrus (SCG; Fig. 1).
Figure 1. Sulcal patterns on the medial surface for the left hemispheres of four cases. The double parallel pattern differs from the single cingulate suclus (cgs) in the elaboration of the paracingulate sulcus (pcgs). When there are just of few short branches of the pcgs, a superior cingulate gyrus (SCG) cannot be identified, however, when the pcgs is fully developed, the double parallel pattern is formed. Tertiary sulci in the ACC tend to lie parallel to the cas as shown by the short and curved arrows in A. In contrast, tertiary sulci in pcgs are vertically oriented. Many of these sulci project directly from the cas (asterisks) and some are free (short, straight arrows). There are many connections between various sulci, however, none appear to be consistent. For example, the splenial sulci (spls) can connect with the cgs (A.), the srs can connect with the pcgs (C. curved arrow), and the pcgs and cgs can attach (D.). The stars around each spls identify the parasplenial sulci.
The presence or absence of the pcgs is the primary basis for defining the single and double parallel sulcal patterns. In light of this variability, Paus et al. (1996) generated probabilistic maps for each hemisphere in 247 individuals and observed that the pcgs is more frequent in the left than right hemispheres. Ide et al. (1999) confirmed this observation by showing a significant hemispheric difference in the incidence of these two sulcal patterns. The single sulcus is more frequent in the right (69%) than left (31%) hemispheres, while the double-parallel pattern is more frequent in the left (68%) than right (32%) hemispheres. Finally, Yücel et al. (2000) showed that males have a greater sulcal asymmetry in the left hemisphere with more prominent pcgs in 20% of males than is true for females who had a prominent pcgs in only 11% of left hemispheres.
The patterns of sulci in the posterior cingulate and caudomedial subregion are also quite variable. The angle or knee at the cgs and inflection of the marginal ramus (mr) is probably one of the deepest points in the cgs. The spls usually has two main vertical/oblique branches that are connected to form an “H” that can itself be connected to the cgs (Fig. 2A). The “H” form occurs in about 65% of spls in right or left hemispheres (Ono et al., 1990), but many other variations have been reported including two or three branches projecting dorsally (Fig. 2D), connections with the cgs (Fig. 2A, B, D), free side branches, and branches that connect with the callosal sulcus (Fig. 2C, D). In light of the many vertical sulci, some projecting from the cas into the posterior cingulate gyrus, the specific morphology of the spls is of little note. Vertical sulci are a feature of most cases and they provide for numerous interconnections between the cas, spls, and cgs. The patterns of these many vertical sulci results in a relatively stable total cortical volume in this region. Brodmann (1909) used a case in which the spls had a typical pattern and showed that area 31 completely surrounds it. The vertical branches of this sulcus, however, are variable in length and can extend beyond the limits of area 31 to penetrate area 23b and sometimes area 23a. Thus, although all cases have a clear though variable Spls, it does not mark the limits of particular cytoarchitectural areas. Indeed, the only gross structural feature in posterior cingulate cortex that consistently delineates a cytoarchitectural border is the cas at the apposition of the ventral apex of the CG and the cc where the border occurs between areas 23a and 30.
The presence or absence of the pcgs is the primary basis for defining the single and double parallel sulcal patterns. In light of this variability, Paus et al. (1996) generated probabilistic maps for each hemisphere in 247 individuals and observed that the pcgs is more frequent in the left than right hemispheres. Ide et al. (1999) confirmed this observation by showing a significant hemispheric difference in the incidence of these two sulcal patterns. The single sulcus is more frequent in the right (69%) than left (31%) hemispheres, while the double-parallel pattern is more frequent in the left (68%) than right (32%) hemispheres. Finally, Yücel et al. (2000) showed that males have a greater sulcal asymmetry in the left hemisphere with more prominent pcgs in 20% of males than is true for females who had a prominent pcgs in only 11% of left hemispheres.
The patterns of sulci in the posterior cingulate and caudomedial subregion are also quite variable. The angle or knee at the cgs and inflection of the marginal ramus (mr) is probably one of the deepest points in the cgs. The spls usually has two main vertical/oblique branches that are connected to form an “H” that can itself be connected to the cgs (Fig. 2A). The “H” form occurs in about 65% of spls in right or left hemispheres (Ono et al., 1990), but many other variations have been reported including two or three branches projecting dorsally (Fig. 2D), connections with the cgs (Fig. 2A, B, D), free side branches, and branches that connect with the callosal sulcus (Fig. 2C, D). In light of the many vertical sulci, some projecting from the cas into the posterior cingulate gyrus, the specific morphology of the spls is of little note. Vertical sulci are a feature of most cases and they provide for numerous interconnections between the cas, spls, and cgs. The patterns of these many vertical sulci results in a relatively stable total cortical volume in this region. Brodmann (1909) used a case in which the spls had a typical pattern and showed that area 31 completely surrounds it. The vertical branches of this sulcus, however, are variable in length and can extend beyond the limits of area 31 to penetrate area 23b and sometimes area 23a. Thus, although all cases have a clear though variable Spls, it does not mark the limits of particular cytoarchitectural areas. Indeed, the only gross structural feature in posterior cingulate cortex that consistently delineates a cytoarchitectural border is the cas at the apposition of the ventral apex of the CG and the cc where the border occurs between areas 23a and 30.
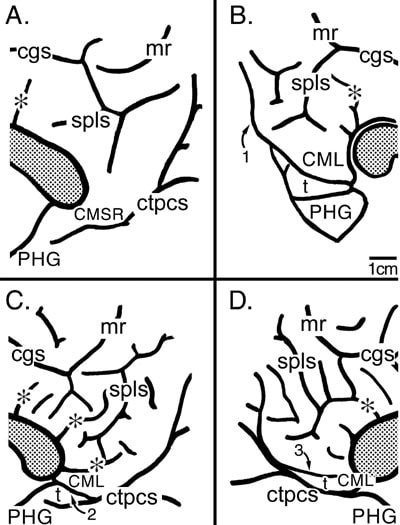
Figure 2. The perisplenial surface for four cases. Asterisks identify some tertiary sulci that project directly from the cas and the splenium of the corpus callosum is shaded. A. The posterior cingulate gyrus is continuous with the parahippocampal gyrus (PHG) and the caudomedial subregion (CMSR) is noted along this transition but there is no identifying feature for the border between cingulate and parahippocampal areas. B. Cortex below the ctpcs was removed at the arrow shown with #1 and this exposed the CML and a transitional PHG (t) that contains the posterior divisions of the parahippocampal area 36' and the PHG can be observed ventral to the PHGt. C. Without dissection of the ventral bank of the ctpcs, the CML, PHGt (t), and PHG can be observed and the PHGt is formed by a branch of the ctpcs and is noted with the #2. D. The CML is formed by the ctpcs, however, the PHGt (t) is formed by a postsplenial dimple marked here with the #3 and the posterior parahippocampal areas are located ventral to this dimple.
The surface of the posterior cingulate gyrus (PCG) extends ventrally to form an isthmus with the posterior parahippocampal cortex and the variable structure of this region is shown in Figure 2. In one case a tongue of cortex provides no landmarks for transition from posterior cingulate cortex to the parahippocampal gyrus (PHG) and the junctional cortex is simply termed the caudomedial subregion (CMSR; Fig. 2A). In another case, a clear fold of tissue at the terminal part of the PCG may not be observed unless the ventral bank of the common truck is removed as at “1" (Fig. 2B). Upon removal of the ventral cortex, the ventral border of a lobule is noted as a transitional fold of cortex termed the transitional PHG (PHGt or “t” in Fig. 2). Goldman-Rakic et al. (1984) referred to this consistent and terminal part of the monkey cingulate gyrus as the caudomedial lobule (CML). When a CML is present in human cases, the common trunk of the parieto-occipital and calcarine sulci (ctpos) often appears to interrupt the isthmus to form the CML (Fig. 2B). In other cases a branch of the ctpos extends to the cas to form a CML (“2” in Fig. 2C is the caudomedial branch of the ctpos). The PHGt lies ventral to the CML, in this instance, where it is formed by the terminal branch of the ctpos. In a fourth variant of CMSR, a postsplenial dimple (“3", Fig. 2D) extends into the CML and defines the border between posterior cingulate cortex and the transitional parahippocampal areas.
The surface of the posterior cingulate gyrus (PCG) extends ventrally to form an isthmus with the posterior parahippocampal cortex and the variable structure of this region is shown in Figure 2. In one case a tongue of cortex provides no landmarks for transition from posterior cingulate cortex to the parahippocampal gyrus (PHG) and the junctional cortex is simply termed the caudomedial subregion (CMSR; Fig. 2A). In another case, a clear fold of tissue at the terminal part of the PCG may not be observed unless the ventral bank of the common truck is removed as at “1" (Fig. 2B). Upon removal of the ventral cortex, the ventral border of a lobule is noted as a transitional fold of cortex termed the transitional PHG (PHGt or “t” in Fig. 2). Goldman-Rakic et al. (1984) referred to this consistent and terminal part of the monkey cingulate gyrus as the caudomedial lobule (CML). When a CML is present in human cases, the common trunk of the parieto-occipital and calcarine sulci (ctpos) often appears to interrupt the isthmus to form the CML (Fig. 2B). In other cases a branch of the ctpos extends to the cas to form a CML (“2” in Fig. 2C is the caudomedial branch of the ctpos). The PHGt lies ventral to the CML, in this instance, where it is formed by the terminal branch of the ctpos. In a fourth variant of CMSR, a postsplenial dimple (“3", Fig. 2D) extends into the CML and defines the border between posterior cingulate cortex and the transitional parahippocampal areas.
References
Azmitia, E.C. and Segal, M. (1978). An autoradiographic analysis of the differential ascending projectionsof the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641-668.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109, 219-233.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. (1979b). Pigment architecture of the human telencephalic cortex. V. Regio anterogenualis. Cell Tissue Res. 204, 441-451.
Broca, P. (1878). Anatomic comparée des circonvolutions cérébrales. Le grand lobe limbique et la scissure limbique dans la série des mammiféres. Rev. Anthropol. 1, Ser 2, 456-498.
Brodmann, K. (1909). "Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues." Barth, Leipzig.
Goldman-Rakic, P.S., Selemon, L.D., and Schwartz, M.L. (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12, 719-743.
Ide, A., Dolezal, C., Fernández, M., Labbé, E., Mandujano, R., Montes, S., Segura, P., Verschae, G., Yarmuch, P., and Aboitiz, F. (1999). Hemispheric differences in variability of fissural patterns in parasylvian and cingulate regions of human brains. J. Comp. Neurol. 410, 235-242.
MacLean, P.D. (1993). Perspectives on Cingulate Cortex in the Limbic System. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 1-15. Birkhäuser, Boston.
Nauta, W. J. H. (1964). Some efferent connections of the prefrontal cortex in the monkey. In "The Frontal Granular Cortex and Behavior" (J. M. Warren and K. Akert, Eds.), pp. 397-409. McGraw-Hill, New York.
Ono, M., Kubik, S., and Abernathey, C.D. (1990). "Atlas of the Cerebral Sulci." Georg Thieme Verlag, New York.
Paus, T., Tomaiuolo, F., Otaky, N., MacDonald, D., Petrides, M., Atlas, J., Morris, R., and Evans, A.C. (1996). Human cingulate and paracingulate sulci: Pattern, variability, asymmetry, and probabilistic map. Cereb. Cortex 6, 207-214.
Retzius, G. (1896). "Das Menschenhirn. Studien in der makroskopischen Morphologie." Norstedt Soner, Stockholm.
Vogt, B.A., Finch, D. M., and Olson, C.R. (1992). Functional heterogeneity of cingulate cortex: The anterior executive and posterior evaluative regions. Cereb. Cortex 2, 435-443.
Vogt, B.A., Nimchinsky, E.A., and Hof, P.R. (1997). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Yücel, M., Stuart, G.W., Maruff, P., Velakoulis, D., Crowe, S., Savage, G., and Pantelis, C. (2000). Hemisphere and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb. Cortex 10, 2-22.
Azmitia, E.C. and Segal, M. (1978). An autoradiographic analysis of the differential ascending projectionsof the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641-668.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Res. 109, 219-233.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. (1979b). Pigment architecture of the human telencephalic cortex. V. Regio anterogenualis. Cell Tissue Res. 204, 441-451.
Broca, P. (1878). Anatomic comparée des circonvolutions cérébrales. Le grand lobe limbique et la scissure limbique dans la série des mammiféres. Rev. Anthropol. 1, Ser 2, 456-498.
Brodmann, K. (1909). "Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues." Barth, Leipzig.
Goldman-Rakic, P.S., Selemon, L.D., and Schwartz, M.L. (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12, 719-743.
Ide, A., Dolezal, C., Fernández, M., Labbé, E., Mandujano, R., Montes, S., Segura, P., Verschae, G., Yarmuch, P., and Aboitiz, F. (1999). Hemispheric differences in variability of fissural patterns in parasylvian and cingulate regions of human brains. J. Comp. Neurol. 410, 235-242.
MacLean, P.D. (1993). Perspectives on Cingulate Cortex in the Limbic System. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 1-15. Birkhäuser, Boston.
Nauta, W. J. H. (1964). Some efferent connections of the prefrontal cortex in the monkey. In "The Frontal Granular Cortex and Behavior" (J. M. Warren and K. Akert, Eds.), pp. 397-409. McGraw-Hill, New York.
Ono, M., Kubik, S., and Abernathey, C.D. (1990). "Atlas of the Cerebral Sulci." Georg Thieme Verlag, New York.
Paus, T., Tomaiuolo, F., Otaky, N., MacDonald, D., Petrides, M., Atlas, J., Morris, R., and Evans, A.C. (1996). Human cingulate and paracingulate sulci: Pattern, variability, asymmetry, and probabilistic map. Cereb. Cortex 6, 207-214.
Retzius, G. (1896). "Das Menschenhirn. Studien in der makroskopischen Morphologie." Norstedt Soner, Stockholm.
Vogt, B.A., Finch, D. M., and Olson, C.R. (1992). Functional heterogeneity of cingulate cortex: The anterior executive and posterior evaluative regions. Cereb. Cortex 2, 435-443.
Vogt, B.A., Nimchinsky, E.A., and Hof, P.R. (1997). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Yücel, M., Stuart, G.W., Maruff, P., Velakoulis, D., Crowe, S., Savage, G., and Pantelis, C. (2000). Hemisphere and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb. Cortex 10, 2-22.