Cingulate Gyrus: Differentiation in Posterior Cingulate Gyrus
The cerebral cortex is comprised of structures with numerous structural gradations. Progressive differences in cortical structure likely reflect developmental and connectional patterns of organization. Since posterior cingulate cortex lies between the single layered indusiuum griseum/subiculum and the isocortical parietal and occipital association areas, it is a region that undergoes radical differentiation. In this context, structural descriptions of a cortical region are most helpful when couched in terms of fundamental patterns of organization rather than only the unique features of a particular area. For example, area 29 has fewer than 6 layers and simply counting from I to IV does not inform which layers contain large ganglionic neurons similar to those in layer V nor which layers contain primarily corticocortical projection neurons and are similar to layer III. Although one might speculate that such layers have no continuity or relationship with those in isocortex, this does not appear to be the case. Indeed, clear relationships are possible through observations of the differentiation of each layer in relation to that in adjacent layers and because each cortical layer has a unique pattern of embryonic differentiation and characteristic connections.
From a developmental perspective, Nowakowski and Rakic (1981) showed that neurons in the hippocampus and deep layers of entorhinal cortex are generated in the subventricular zone. Thus, neurons throughout the indusium griseum and deep layers of the ventral bank of the cingulate gyrus can be expected to share in a similar origin. Furthermore, the projections of layer VI neurons throughout the cingulate gyrus and including the retrosplenial areas are to the thalamus as shown in the monkey (Baleydier and Mauguiere, 1985). Thus, the deep layers of retrosplenial cortex share similarities in origin and projections that allow one to conclude that they are part of a similar differentiation scheme. Indeed, the concept of cortical differentiation is based on the principle that a common motif can be identified for all cortical areas such as layer V. It is upon this basic substratum that cortical differentiation proceeds to culminate in a fully elaborated isocortex.
From a developmental perspective, Nowakowski and Rakic (1981) showed that neurons in the hippocampus and deep layers of entorhinal cortex are generated in the subventricular zone. Thus, neurons throughout the indusium griseum and deep layers of the ventral bank of the cingulate gyrus can be expected to share in a similar origin. Furthermore, the projections of layer VI neurons throughout the cingulate gyrus and including the retrosplenial areas are to the thalamus as shown in the monkey (Baleydier and Mauguiere, 1985). Thus, the deep layers of retrosplenial cortex share similarities in origin and projections that allow one to conclude that they are part of a similar differentiation scheme. Indeed, the concept of cortical differentiation is based on the principle that a common motif can be identified for all cortical areas such as layer V. It is upon this basic substratum that cortical differentiation proceeds to culminate in a fully elaborated isocortex.
Transition in Cortical Structure
Each area on the cingulate gyrus is transitional in nature. That is to say, every area shares cytoarchitectural features of its two or more adjacent areas. When there are transitions between cingulate and adjacent frontal or parietal cortices, the term transition designates the cingulofrontal transitional area 32 and the cinguloparietal transitional area 31, respectively. Rather than speak in terms of transition from the perspective of single areas, we use this concept in relation to broad trends of cortical differentiation. Many students of cytoarchitecture have considered cortical trends of differentiation in the cingulate gyrus (von Economo and Koskinas, 1925; Braak, 1979a; Braak and Braak, 1993) and this includes progressive alterations throughout the ectosplenial, retrosplenial and posterior cingulate regions. Differentiation begins with a substratum of densely packed neurons in the internal pyramidal layer. This is followed by the addition and differentiation of a granular external pyramidal and ends in the parietal and occipital association areas. A second trend includes the rostral agranular cortex and begins with the ectogenual areas and progresses through a differentiation patterns leading to the cingulate motor and supplementary motor areas. Since it is the former of these two trends for which there is most information, this trend is considered here in detail.
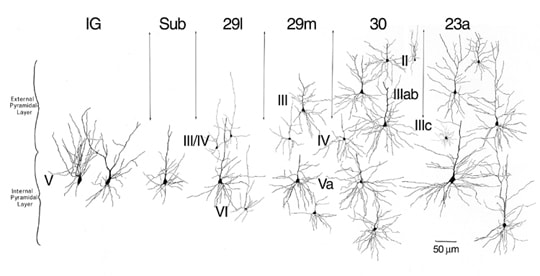
A study of the somatic and dendritic architecture of neurons in the retrosplenial region led to a conceptualization of the progressive changes of neuronal architecture in this region (Vogt, 1976) and it is shown modified for the present terminology in Figure 1. The density of large ganglionic neurons in the IG is so high that the apical and basal dendritic trees of these neurons stretch in a bipolar fashion from the apical and basal parts of the somata. In the subiculum, the basal dendritic tree is elaborated in a horizontal orientation and the density of neurons is reduced. In the retrosplenial areas the progressive elaboration of the basal dendritic tree increases along with reductions in neuron density until the largest neurons in layer Va of areas 23a and 23b are attained with their equally extensive basal dendritic tree. Schlaug et al. (1994) considered the relationship between basal dendritic tree elaboration and cell density in layer V along a different dimension but came to a similar conclusion. They observed that the progression from anterior to posterior cingulate cortex on the gyral surface was associated with a reduction in the extent of the basal dendritic tree and there was a progressive increase in the density of neurons. Thus, it is a general principle that, as basal dendrites are elaborated, there is a commensurate reduction in the density of neurons.
A study of the somatic and dendritic architecture of neurons in the retrosplenial region led to a conceptualization of the progressive changes of neuronal architecture in this region (Vogt, 1976) and it is shown modified for the present terminology in Figure 1. The density of large ganglionic neurons in the IG is so high that the apical and basal dendritic trees of these neurons stretch in a bipolar fashion from the apical and basal parts of the somata. In the subiculum, the basal dendritic tree is elaborated in a horizontal orientation and the density of neurons is reduced. In the retrosplenial areas the progressive elaboration of the basal dendritic tree increases along with reductions in neuron density until the largest neurons in layer Va of areas 23a and 23b are attained with their equally extensive basal dendritic tree. Schlaug et al. (1994) considered the relationship between basal dendritic tree elaboration and cell density in layer V along a different dimension but came to a similar conclusion. They observed that the progression from anterior to posterior cingulate cortex on the gyral surface was associated with a reduction in the extent of the basal dendritic tree and there was a progressive increase in the density of neurons. Thus, it is a general principle that, as basal dendrites are elaborated, there is a commensurate reduction in the density of neurons.
Figure 1. Neuronal characteristics of transition in the macaque monkey include a progressive differentiation of layers, addition of layers, and a reduction in neuron densities associated with elaboration of the basal dendritic tree. This reconstruction of Golgi impregnated neurons is from the ventral bank of the cingulate gyrus (Vogt, 1976) and has been modified for the current nomenclature. Progressive differentiation of the basal dendritic tree of pyramidal neurons is prominent in layer V of all areas, layer III/IV is added in area 29l, layer III is added in area 29m, and layer II appears in medial parts of area 30. Area 23a is a fully differentiated isocortical structure.
In area 29l, the external pyramidal layer of small and granular neurons is added to the ganglionic layer of the subicular rudiment. This layer is termed layer III/IV for reasons discussed above. In area 29m there is a layer of medium-sized pyramidal neurons added superficial to the granular layer IV which is termed layer III and layer V differentiates into a layers Va and Vb. Layer III then differentiates into layers IIIab and IIIc in area 30. At the medial border of area 30 a layer II becomes apparent which is characteristic of isocortical areas. Finally, the dysgranular character of layer IV in area 30 is altered in area 23a such that layer IV is a continuous and granular. Each of these patterns observed in monkey can be demonstrated immunohistochemically in human retrosplenial cortex.
In area 29l, the external pyramidal layer of small and granular neurons is added to the ganglionic layer of the subicular rudiment. This layer is termed layer III/IV for reasons discussed above. In area 29m there is a layer of medium-sized pyramidal neurons added superficial to the granular layer IV which is termed layer III and layer V differentiates into a layers Va and Vb. Layer III then differentiates into layers IIIab and IIIc in area 30. At the medial border of area 30 a layer II becomes apparent which is characteristic of isocortical areas. Finally, the dysgranular character of layer IV in area 30 is altered in area 23a such that layer IV is a continuous and granular. Each of these patterns observed in monkey can be demonstrated immunohistochemically in human retrosplenial cortex.
Dysgranular Concept and Transition
At each point of transition from allocortex to isocortex throughout the primate telencephalon, there is at least one area that has the features of a dysgranular cortex and this is why the character of area 30 is so important. In the insula, neurons in layer IV form islands as is characteristic of a layer that is of irregular thickness (Mufson et al., 1997), while orbitofrontal cortex has an intermediate area with a thin layer IV which can be difficult to detect where the layer III and V pyramids are particularly large (Hof et al., 1995).
Brodmann (1909) referred to area 30 as agranular and von Economo and Koskinas (1925) were quite explicit that area LD is not just agranular but that the “granulous” layer of area LE (Brodmann’s area 29) is not continuous with the isocortical layer of area LC2 (Brodmann’s area 23). Although there is no doubt that layer III/IV in area 29 is not equivalent to layer IV in area 23a, the following three points need to be considered. First, von Economo (1929; Fig. 50) vacillated on the presence of a layer IV in LD and showed a layer III(IV) below layer III therein. Second, Figure 2 shows that the granular layer IV of area 29m is continuous with layer IV in area 30 and layer IV in area 23a. This does not mean that layer IV in each area is equivalent, however, von Economo’s statement that they are not continuous and not supported because immunohistochemical preparations show this continuity as emphasized below. Finally, the dysgranular concept for a cortical architecture was not established during the early years of cytoarchitectonic analysis nor were immunohistochemical techniques available to clarify the concept. It is not surprising, therefore, that the dysgranular structure of area 30/LD was not appreciated by early neuroanatomists.
The dysgranular nature of area 30 in monkey and human brains has been documented with Nissl, Golgi, and immunohistochemical techniques (Vogt, 1976; Vogt et al., 1995, 1997). Layer IV is of variable thickness with points at which layers IIIc and Va join via neuronal bridges across layer IV and a recent study demonstrated the dysgranular structure of layer IV in RSC using photomontages along its full lateromedial extent in area 30 (Vogt et al., 2001). Another example of a dysgranular cortex is in anterior cingulate cortex where area 32, which sits at the juncture of anterior cingulate and prefrontal cortices, was shown to have islands of neurons in layer IV (Vogt et al., 1995).
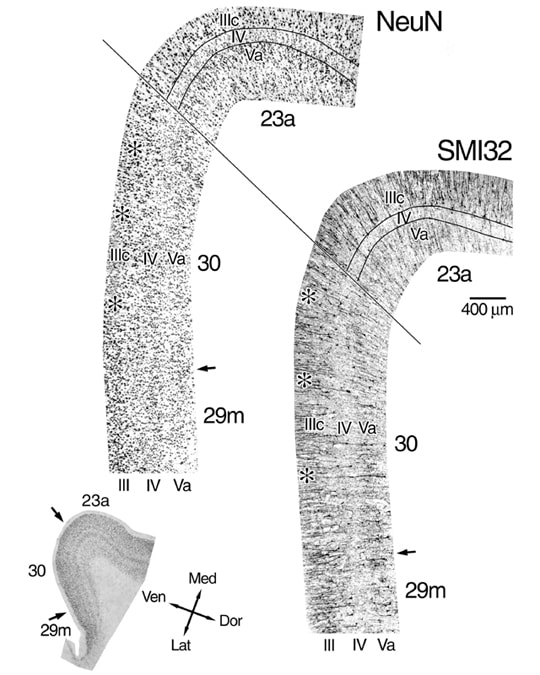
It is difficult to document dysgranular cortex in single strips of cortex because the structure of layer IV is so variable. Therefore, Figure 2 shows all of layer IV at a relatively high magnification in both NeuN and SMI32 preparations of area 30. The NFP-ir neuronal plexuses in layers III and V show via a negative image for layer IV that layer IV is continuous throughout area 30. Since most layer IV neurons are not immunoreactive and there are no glial or vascular elements stained, this preparation emphasizes the continuity of layer IV. The NeuN preparation shows that there are small neurons in layer IV and that there are breaks in layer IV by medium and large pyramidal neurons. The bridges of large pyramids between layers IIIc and Va are noted with asterisks. The profound differences between the architectures of dysgranular area 30 and granular area 23a are also clear in Figure 29. Thus, the dysgranular characteristics of area 30 are similar to those described previously for dysgranular areas in orbitofrontal, insular, and cingulofrontal transition areas (Hof et al., 1995; Mufson et al., 1997; Vogt et al., 1995).
Brodmann (1909) referred to area 30 as agranular and von Economo and Koskinas (1925) were quite explicit that area LD is not just agranular but that the “granulous” layer of area LE (Brodmann’s area 29) is not continuous with the isocortical layer of area LC2 (Brodmann’s area 23). Although there is no doubt that layer III/IV in area 29 is not equivalent to layer IV in area 23a, the following three points need to be considered. First, von Economo (1929; Fig. 50) vacillated on the presence of a layer IV in LD and showed a layer III(IV) below layer III therein. Second, Figure 2 shows that the granular layer IV of area 29m is continuous with layer IV in area 30 and layer IV in area 23a. This does not mean that layer IV in each area is equivalent, however, von Economo’s statement that they are not continuous and not supported because immunohistochemical preparations show this continuity as emphasized below. Finally, the dysgranular concept for a cortical architecture was not established during the early years of cytoarchitectonic analysis nor were immunohistochemical techniques available to clarify the concept. It is not surprising, therefore, that the dysgranular structure of area 30/LD was not appreciated by early neuroanatomists.
The dysgranular nature of area 30 in monkey and human brains has been documented with Nissl, Golgi, and immunohistochemical techniques (Vogt, 1976; Vogt et al., 1995, 1997). Layer IV is of variable thickness with points at which layers IIIc and Va join via neuronal bridges across layer IV and a recent study demonstrated the dysgranular structure of layer IV in RSC using photomontages along its full lateromedial extent in area 30 (Vogt et al., 2001). Another example of a dysgranular cortex is in anterior cingulate cortex where area 32, which sits at the juncture of anterior cingulate and prefrontal cortices, was shown to have islands of neurons in layer IV (Vogt et al., 1995).
It is difficult to document dysgranular cortex in single strips of cortex because the structure of layer IV is so variable. Therefore, Figure 2 shows all of layer IV at a relatively high magnification in both NeuN and SMI32 preparations of area 30. The NFP-ir neuronal plexuses in layers III and V show via a negative image for layer IV that layer IV is continuous throughout area 30. Since most layer IV neurons are not immunoreactive and there are no glial or vascular elements stained, this preparation emphasizes the continuity of layer IV. The NeuN preparation shows that there are small neurons in layer IV and that there are breaks in layer IV by medium and large pyramidal neurons. The bridges of large pyramids between layers IIIc and Va are noted with asterisks. The profound differences between the architectures of dysgranular area 30 and granular area 23a are also clear in Figure 29. Thus, the dysgranular characteristics of area 30 are similar to those described previously for dysgranular areas in orbitofrontal, insular, and cingulofrontal transition areas (Hof et al., 1995; Mufson et al., 1997; Vogt et al., 1995).
Figure 2. The dysgranular nature of area 30 demonstrated with full reconstructions of layer IV (montages of 7 photographs) rather than single cortical strips because of the incipient nature of this layer. Each montage is separated by about 300 µm and are from the level shown below. A continual layer IV appears in the SMI32 preparation because of the negative staining image; i.e., neurons that are NFP-ir in this layer are much smaller than those in layers IIIc and Va and the plexus of these dendrites is diffuse. Variability in layer IV thickness is emphasized with asterisks at points where neurons from layers IIIc and Va intermingle. The borders of layer IV are noted for area 23a because the layer is uniform and uninterrupted, while a similar outline was not made in area 30 to avoid obliterating critical morphology.
Retrosplenial Differentiation Pattern in Human
The pattern of retrosplenial cortical differentiation is as obvious in the human as it is in the macaque monkey. The SMI32 preparations are particularly useful in demonstrating these patterns of change because the large pyramidal neurons are so often NFP-ir in this region and these preparations eliminates glial and small neuronal elements. To the extent that small neurons in layers II and IV also contribute to the patterns of differentiation, the NeuN preparations make an important companion for such an analysis. Figure 3 provides a series of photographs through each area on the ventral bank of the cingulate sulcus that are aligned at the border of layers III/IV and V or layers IV and V. The cell dense layer of the IGr and subiculum are NFP-ir. The addition of layer III/IV in area 26 is as prominent a change as is the overall reduction in the number of neurons in the deep layer. In area 29l, layer III/IV becomes somewhat less neuron dense and the neurons are larger. The deep layer V is also populated by many more large and NFP-ir neurons than is true for area 26. A number of radical changes in morphology occur in area 29m including differentiation of a thick layer of medium to large pyramids, definition of a layer IV and elaboration of layer Va. Some of the neurons in layer III are so large in the medial part of area 29m that they may presage those of layer IIIc in area 30 as shown in Figure 30 at the asterisk. Finally, layer III differentiates further in area 30 and in area 23a layer IV thickens to form an isocortical granular layer.
Figure 3. Cortical transition in human shown for large, NFT-ir neurons. Some of the changes noted in monkey occur in human but there is the addition of area 26 with its external granular layer and only a modest number of pyramids in deep layers. Differentiation of layer III/IV into layers III and IV occurs in area 29m. The asterisk marks the medial part of area 29m where a few large layer IIIc pyramids are first observed. The arrows in superficial layers draw the eye through the transitional events in areas 29m, 30, and 23a.
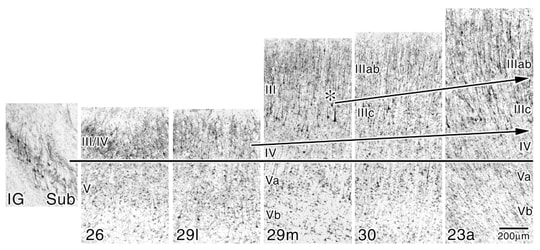
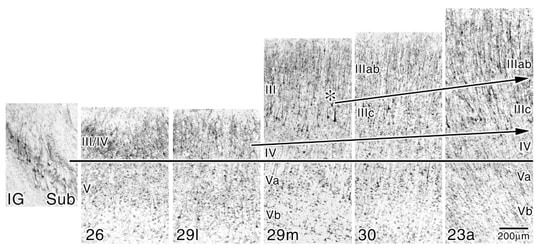
The patterns of layers II and IV elaboration are evident in the NeuN preparations where all neurons are immunoreactive rather than just a subset of large, NFP-ir pyramidal neurons in the SMI32 preparations. Figure 4 shows each of the ectosplenial and retrosplenial areas aligned at the top of layer Va. The progressive elaboration of layer III/IV is evident. It is even possible to identify a layer IV in area 29l which we have not done previously. It appears this differentiation is possible in Figure 4 because a higher level of contrast was applied to the photographic reproduction to further highlight neuronal somata. A parvicellular layer is clear in area 29m and it differentiates further in areas 30 and 23a. The demonstration of layer II addition is made by selecting the point in area 30 where layer II first appears as indicated with the asterisk. The lancet shape of these neurons appears in area 23a where layer IIIc also reaches a high degree of differentiation in contrast to the plump layer II neurons in area 30. The highest level of differentiation of layers IIIc and IV occurs in area 31 which is not shown in this figure, however, an SMI32 preparation of area 31 is magnified in a figure in “Cytology” and noted below. Thus, a combination of immunohistochemical techniques provides a compelling view of different aspects of cortical transition in the human retrosplenial region and provides a rationale for the nomenclature of each layer.
The patterns of layers II and IV elaboration are evident in the NeuN preparations where all neurons are immunoreactive rather than just a subset of large, NFP-ir pyramidal neurons in the SMI32 preparations. Figure 4 shows each of the ectosplenial and retrosplenial areas aligned at the top of layer Va. The progressive elaboration of layer III/IV is evident. It is even possible to identify a layer IV in area 29l which we have not done previously. It appears this differentiation is possible in Figure 4 because a higher level of contrast was applied to the photographic reproduction to further highlight neuronal somata. A parvicellular layer is clear in area 29m and it differentiates further in areas 30 and 23a. The demonstration of layer II addition is made by selecting the point in area 30 where layer II first appears as indicated with the asterisk. The lancet shape of these neurons appears in area 23a where layer IIIc also reaches a high degree of differentiation in contrast to the plump layer II neurons in area 30. The highest level of differentiation of layers IIIc and IV occurs in area 31 which is not shown in this figure, however, an SMI32 preparation of area 31 is magnified in a figure in “Cytology” and noted below. Thus, a combination of immunohistochemical techniques provides a compelling view of different aspects of cortical transition in the human retrosplenial region and provides a rationale for the nomenclature of each layer.
Figure 4. High contrast prints of NeuN-immunoreacted tissue show changes in the superficial layers of the human retrosplenial areas not evident in SMI32 preparations of the previous figure; particularly layers II, IIIab, and IV that have few or no NFP-ir neurons. The rationale for not terming the granular layer of areas 26 and 29 layer II-IV is apparent; layer II is not present until the medial part of area 30 (asterisk; II). Differentiation of the superficial layers in terms of neuron densities and cell shapes is noteworthy in layer II where the plump vs lancet-shaped pyramids of areas 30 and 23a, respectively, are shown. Arrows are oriented to emphasize the direction of cortical transition and do not always fall on each laminar border.
Area 31: Pinnacle of Cingulate Isocortical Differentiation
Area 31 represents the culmination of two important trends in cingulate cortical transition. The first differentiation trend is that of layer IIIc with a unique neuronal phenotype and it is observed in the rostrocaudal dimension on the cingulate gyrus. Beginning with the anterior region there is a progressive increase in the number of large layer III pyramidal neurons. Area 24 has only an undifferentiated layer III, area 24' has more large neurons in this layer with progressively higher numbers of NFP-ir neurons but there is only a decrease in neuron density and not a well defined layer IIIc with large pyramidal neurons. Area 23 has a fully developed layer IIIc and a moderate to high density of NFP-ir neurons in this layer. Area 31 caps this trend with the most extensive layer IIIc and the highest density of NFP-ir neurons on the medial surface. These neurons are shown in an incert for Figure 17 in the section on “Cytology.”
The second differentiation trend is that of layer IV which occurs in the ventrodorsal dimension on the cingulate gyrus. This trend starts with layer III/IV of area 26. Layer III/IV of areas 26 and 29l differentiate into layers III and IV in area 29m, while in area 30 there is the dysgranular layer IV. In area 23 layer IV reaches the isocortical thickness, but in area 31, layer IV is at the maximal thickness and overall neuron density. Thus, area 31 is the final and most differentiated form of granular cingulate cortex and it forms the superior and caudal parts of the posterior cingulate gyrus.
The second differentiation trend is that of layer IV which occurs in the ventrodorsal dimension on the cingulate gyrus. This trend starts with layer III/IV of area 26. Layer III/IV of areas 26 and 29l differentiate into layers III and IV in area 29m, while in area 30 there is the dysgranular layer IV. In area 23 layer IV reaches the isocortical thickness, but in area 31, layer IV is at the maximal thickness and overall neuron density. Thus, area 31 is the final and most differentiated form of granular cingulate cortex and it forms the superior and caudal parts of the posterior cingulate gyrus.
References
Baleydier, C. and Mauguiere, F. (1985). Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J. Comp . Neurol. 232, 219-228.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. and Braak, E. (1993). Alzheimer Neuropathology and Limbic Circuits. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 606-626. Birkhäuser, Boston.
Brodmann, K. (1909). "Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues." Barth, Leipzig.
von Economo, C. and Koskinas, G. N. (1925). "Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen." Springer, Berlin.
von Economo, C. (1929). "The Cytoarchitectonics of the Human Cerebral Cortex." Oxford University Press, London
Hof, P. R., Mufson, E. J., and Morrison, J. H. (1995). Human orbitofrontal cortex cytoarchitecture and quantitative immunohistochemical parcellation. J. Comp. Neurol. 359, 48-68.
Mufson, E. J., Sobreviela, T., and Kordower, J. H. (1997). Chemical neuroanatomy of the primate insula cortex: relationship to cytoarchitectonics, connectivity function, and neurodegeneration. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, Elsevier, Amsterdam.
Nowakowski, R. S. and Rakic, P. (1981). The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J. Comp. Neurol. 196, 129-154.
Schlaug, G., Armstrong, E., Schleicher, A., and Zilles, K. (1993). Layer V pyramidal cells in the adult human cingulate cortex. Anat. Embryol. 187, 515-522.
Schlaug, G., Knorr, U., and Seitz, R. J. (1994). Inter-subject variability of cerebral activations in acquiring a motor skill: A study with positron emission tomography. Exp. Brain Res. 98, 523-534.
Vogt, B. A. (1976). Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. J. Comp .Neurol. 169, 63-98.
Vogt, B. A., Nimchinsky, E. A., Vogt, L. J., and Hof, P. R. (1995). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Vogt, B. A., Vogt, L. J., Nimchinsky, E. A., and Hof, P. R. (1997). Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer's disease. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, pp. 455-528, Elsevier, Amsterdam.
Vogt, B. A., Vogt, L. J., Perl, D. P., and Hof, P. R. (2001). Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J. Comp. Neurol. 438:353-376.
Baleydier, C. and Mauguiere, F. (1985). Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J. Comp . Neurol. 232, 219-228.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. and Braak, E. (1993). Alzheimer Neuropathology and Limbic Circuits. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 606-626. Birkhäuser, Boston.
Brodmann, K. (1909). "Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues." Barth, Leipzig.
von Economo, C. and Koskinas, G. N. (1925). "Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen." Springer, Berlin.
von Economo, C. (1929). "The Cytoarchitectonics of the Human Cerebral Cortex." Oxford University Press, London
Hof, P. R., Mufson, E. J., and Morrison, J. H. (1995). Human orbitofrontal cortex cytoarchitecture and quantitative immunohistochemical parcellation. J. Comp. Neurol. 359, 48-68.
Mufson, E. J., Sobreviela, T., and Kordower, J. H. (1997). Chemical neuroanatomy of the primate insula cortex: relationship to cytoarchitectonics, connectivity function, and neurodegeneration. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, Elsevier, Amsterdam.
Nowakowski, R. S. and Rakic, P. (1981). The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J. Comp. Neurol. 196, 129-154.
Schlaug, G., Armstrong, E., Schleicher, A., and Zilles, K. (1993). Layer V pyramidal cells in the adult human cingulate cortex. Anat. Embryol. 187, 515-522.
Schlaug, G., Knorr, U., and Seitz, R. J. (1994). Inter-subject variability of cerebral activations in acquiring a motor skill: A study with positron emission tomography. Exp. Brain Res. 98, 523-534.
Vogt, B. A. (1976). Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. J. Comp .Neurol. 169, 63-98.
Vogt, B. A., Nimchinsky, E. A., Vogt, L. J., and Hof, P. R. (1995). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490-506.
Vogt, B. A., Vogt, L. J., Nimchinsky, E. A., and Hof, P. R. (1997). Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer's disease. In "Handbook of Chemical Neuroanatomy, The Primate Nervous System" (F. E. Bloom, A. Björklund, and T. Hökfelt, Eds.), Vol. 13, Part I, pp. 455-528, Elsevier, Amsterdam.
Vogt, B. A., Vogt, L. J., Perl, D. P., and Hof, P. R. (2001). Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J. Comp. Neurol. 438:353-376.