Rat Cingulate Cortex & Disease Models (3 of 3)
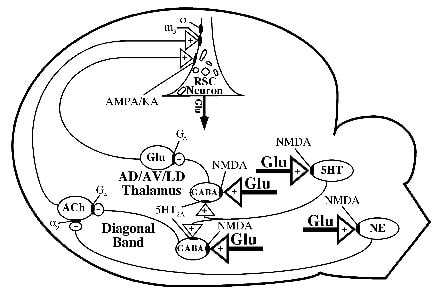
Figure 12. Circuitry mediating NRHypo toxicity. Glu acts via NMDA receptors on GABAergic and noradrenergic (NE) neurons and maintains tonic inhibitory control over two major excitatory pathways that innervate neurons in RSC (areas 29a-c). Systemic administration of NMDA antagonists block NMDA receptors and abolish inhibitory control over excitatory inputs to RSC. The disinhibited excitatory pathways hyper-activate RSC neurons, possibly disrupting multiple intracellular signaling systems, thereby causing immediate derangement of the cognitive functions of RSC and reversible or irreversible neuronal injury, depending upon the length of the exposure. It is postulated that the glutamatergic cell bodies that project to the AMPA/KA receptors in RSC are located in the anterior thalamus. Although this diagram emphasizes RSC, a similar disinhibitory mechanism and similar but not necessarily the same circuits and receptor mechanisms may mediate damage in other regions by sustained NRHypo. Excitatory (+) and inhibitory(-) inputs are shown. ACh, acetylcholine; GA, GABAA receptor; m3, muscarinic receptor (m3 subtype); s, sigma site; 5-HT, serotonin.
Non-NMDA glutamatergic system. While these data confirm that disinhibition of the cholinergic system is a necessary component underlying NRHypo neurotoxicity, it is not sufficient because the injection of carbachol, a muscarinic agonist, directly into the RSC does not reproduce the damage (Farber et al., 2002). NMDA antagonists also produce excessive release of Glu in the cerebral cortex suggesting that excessive stimulation of glutamatergic receptors might also be involved in the neurotoxic process. NBQX, an antagonist of AMPA and KA receptors, protects against NRHypo neurotoxicity, when applied systemically or when injected directly into the RSC (Farber et al., 2002), indicating that the excessively stimulated glutamatergic receptors are likely of the AMPA/KA subtype. Although injection of KA or AMPA directly into the RSC does not reproduce the damage (Farber et al., 2002), co-injection of KA and carbachol does reproduce the neurotoxicity (Farber et al., 2002). The need for both agents to produce the damage indicates that the combined excessive activation of both muscarinic and non-NMDA, glutamatergic receptors is necessary and sufficient to produce the neurotoxicity. Injection of muscimol into either the AD/AV or LD nucleus of the thalamus, where thalamic input into the RSC arises (Figs. 6 and 9), protects against NRHypo neurotoxicity (Jiang et al., 2001), indicating that thalamic glutamatergic neurons are the likely source of the excessive release of Glu in the RSC and that these neurons also are under tonic inhibition from NMDA-receptor bearing GABAergic neurons (Fig 12).
Additional evidence that NMDA antagonists produce disinhibition. Based on this disinhibition model, agents that reduce the ability of these excitatory projections to release excessive neurotransmitter and stimulate the vulnerable RSC neuron should protect against the neurotoxic reaction. Activation of voltage-gated sodium channels is necessary for propagation of the action potential down the axon and inhibitors of these channels, e.g. tetrodotoxin, valproic acid, and carbamazepine, prevent NRHypo neurotoxicity (Farber et al., 2002b). NMDA antagonists also acutely increase metabolism in certain corticolimbic regions (Farber et al., 1999, 2002). In general the corticolimbic regions experiencing hypermetabolism tend to be the same corticolimbic regions that also develop either the reversible or irreversible forms of NRHypo neurotoxicity. The increase in metabolism in these corresponding regions could be a reflection of a disinhibition syndrome in which acetylcholine and Glu are excessively released at certain corticolimbic neurons that are injured in the NRHypo neurotoxic syndrome. Consistent with this proposal, clozapine and halothane reverse the hypermetabolism induced by NMDA antagonists (Duncan et al., 1998b) just as they reverse NRHypo neurotoxicity (Ishimaru et al., 1995; Farber et al., 1996).
Other markers of NRHypo-induced pathology and disinhibition circuit. NRHypo produces several other effects. Dragunow and Faull (1990) reported that MK-801 induced the production of c-Fos protein in these same neurons and, not only c-Fos, but other immediate-early genes, including c-Jun, Jun-B, NGFI-A [a.k.a. zif268, krox-24], NGFI-B, NGFI-C and Nurr1 (Farber and Newcomer, in press), are activated by NRHypo. In addition, the heat shock protein HSP70 and its mRNA are induced by NRHypo (Sharp et al., 1991; Olney et al., 1991). Lastly, NRHypo induces the expression of brain derived growth factor mRNA (Hughes et al., 1993; Castren et al., 1993). The ability of some of the same pharmacological treatments, which have been shown to prevent NRHypo neurotoxicity, to prevent these other responses (Farber and Newcomer, 2002) suggests that these other responses may be secondary to activation of the same NRHypo disinhibition mechanism. Consistent with this proposal, PCP's induction of c-Fos and HSP70 has a similar age-dependency profile (Sharp et al., 1992; Sato et al., 1997), as does MK-801 induction of the reversible form of NRHypo neurotoxicity (Farber et al., 1995b).
Non-NMDA glutamatergic system. While these data confirm that disinhibition of the cholinergic system is a necessary component underlying NRHypo neurotoxicity, it is not sufficient because the injection of carbachol, a muscarinic agonist, directly into the RSC does not reproduce the damage (Farber et al., 2002). NMDA antagonists also produce excessive release of Glu in the cerebral cortex suggesting that excessive stimulation of glutamatergic receptors might also be involved in the neurotoxic process. NBQX, an antagonist of AMPA and KA receptors, protects against NRHypo neurotoxicity, when applied systemically or when injected directly into the RSC (Farber et al., 2002), indicating that the excessively stimulated glutamatergic receptors are likely of the AMPA/KA subtype. Although injection of KA or AMPA directly into the RSC does not reproduce the damage (Farber et al., 2002), co-injection of KA and carbachol does reproduce the neurotoxicity (Farber et al., 2002). The need for both agents to produce the damage indicates that the combined excessive activation of both muscarinic and non-NMDA, glutamatergic receptors is necessary and sufficient to produce the neurotoxicity. Injection of muscimol into either the AD/AV or LD nucleus of the thalamus, where thalamic input into the RSC arises (Figs. 6 and 9), protects against NRHypo neurotoxicity (Jiang et al., 2001), indicating that thalamic glutamatergic neurons are the likely source of the excessive release of Glu in the RSC and that these neurons also are under tonic inhibition from NMDA-receptor bearing GABAergic neurons (Fig 12).
Additional evidence that NMDA antagonists produce disinhibition. Based on this disinhibition model, agents that reduce the ability of these excitatory projections to release excessive neurotransmitter and stimulate the vulnerable RSC neuron should protect against the neurotoxic reaction. Activation of voltage-gated sodium channels is necessary for propagation of the action potential down the axon and inhibitors of these channels, e.g. tetrodotoxin, valproic acid, and carbamazepine, prevent NRHypo neurotoxicity (Farber et al., 2002b). NMDA antagonists also acutely increase metabolism in certain corticolimbic regions (Farber et al., 1999, 2002). In general the corticolimbic regions experiencing hypermetabolism tend to be the same corticolimbic regions that also develop either the reversible or irreversible forms of NRHypo neurotoxicity. The increase in metabolism in these corresponding regions could be a reflection of a disinhibition syndrome in which acetylcholine and Glu are excessively released at certain corticolimbic neurons that are injured in the NRHypo neurotoxic syndrome. Consistent with this proposal, clozapine and halothane reverse the hypermetabolism induced by NMDA antagonists (Duncan et al., 1998b) just as they reverse NRHypo neurotoxicity (Ishimaru et al., 1995; Farber et al., 1996).
Other markers of NRHypo-induced pathology and disinhibition circuit. NRHypo produces several other effects. Dragunow and Faull (1990) reported that MK-801 induced the production of c-Fos protein in these same neurons and, not only c-Fos, but other immediate-early genes, including c-Jun, Jun-B, NGFI-A [a.k.a. zif268, krox-24], NGFI-B, NGFI-C and Nurr1 (Farber and Newcomer, in press), are activated by NRHypo. In addition, the heat shock protein HSP70 and its mRNA are induced by NRHypo (Sharp et al., 1991; Olney et al., 1991). Lastly, NRHypo induces the expression of brain derived growth factor mRNA (Hughes et al., 1993; Castren et al., 1993). The ability of some of the same pharmacological treatments, which have been shown to prevent NRHypo neurotoxicity, to prevent these other responses (Farber and Newcomer, 2002) suggests that these other responses may be secondary to activation of the same NRHypo disinhibition mechanism. Consistent with this proposal, PCP's induction of c-Fos and HSP70 has a similar age-dependency profile (Sharp et al., 1992; Sato et al., 1997), as does MK-801 induction of the reversible form of NRHypo neurotoxicity (Farber et al., 1995b).
NRHypo-Induced Psychosis
A variety of NMDA antagonists (e.g., ketamine, PCP, CPP, CPP-ene, CGS19755, CNS 1102) cause a psychotic state in humans (Farber and Newcomer, in press). These findings suggest that a NRHypo state might be involved in the pathophysiology of psychotic disorders. While schizophrenia has received the most attention as the disorder in which an NRHypo state might exist (e.g. Olney and Farber, 1995), the fact that NMDA antagonists can produce maniacal excitation, catatonic signs and euphoria suggests that such a NRHypo state also could be responsible for some of the signs and symptoms of bipolar and schizoaffective disorder (Farber and Newcomer, 2001).
Based upon several intriguing parallels between NRHypo neurotoxicity and NRHypo-induced psychosis, it has been proposed (Olney and Farber, 1995; Farber et al., 1999, Farber and Newcomer, 2002) that the complex polysynaptic disinhibition mechanism that underlies the neurotoxic action of NMDA antagonists also underlies their psychotomimetic effects. This model proposes that mild elevations in the release of acetylcholine and Glu induced by mild NRHypo result in functional over-activation of cerebrocortical neurons and their projection fields, producing cognitive and behavioral disturbances without neurotoxicity. More severe NRHypo causes greater increases in the amount of excessive transmitter release and in the degree of postsynaptic m3 and non-NMDA receptor over stimulation, resulting in neurotoxicity. While the exact role that a NRHypo-disinhibited state plays in idiopathic psychotic disorders like schizophrenia is mostly hypothetical, the data in rodents point to the importance of NMDA receptor-bearing GABAergic interneurons in certain cortical and thalamic regions. Consistent with this conclusion are reports of deficiencies in GABAergic and NMDA/glutamatergic systems in cortical and thalamic regions of subjects with schizophrenia and other idiopathic psychotic disorders (Woo et al., 1998; Benes, 1999; Guidotti et al., 2000; Ibrahim et al., 2000).
Based upon several intriguing parallels between NRHypo neurotoxicity and NRHypo-induced psychosis, it has been proposed (Olney and Farber, 1995; Farber et al., 1999, Farber and Newcomer, 2002) that the complex polysynaptic disinhibition mechanism that underlies the neurotoxic action of NMDA antagonists also underlies their psychotomimetic effects. This model proposes that mild elevations in the release of acetylcholine and Glu induced by mild NRHypo result in functional over-activation of cerebrocortical neurons and their projection fields, producing cognitive and behavioral disturbances without neurotoxicity. More severe NRHypo causes greater increases in the amount of excessive transmitter release and in the degree of postsynaptic m3 and non-NMDA receptor over stimulation, resulting in neurotoxicity. While the exact role that a NRHypo-disinhibited state plays in idiopathic psychotic disorders like schizophrenia is mostly hypothetical, the data in rodents point to the importance of NMDA receptor-bearing GABAergic interneurons in certain cortical and thalamic regions. Consistent with this conclusion are reports of deficiencies in GABAergic and NMDA/glutamatergic systems in cortical and thalamic regions of subjects with schizophrenia and other idiopathic psychotic disorders (Woo et al., 1998; Benes, 1999; Guidotti et al., 2000; Ibrahim et al., 2000).
NRHypo and Neurodegeneration in Alzheimer’s Disease
One of the first sites of impaired glucose metabolism in Alzheimer’s disease (AD) patients with early memory impairment is in posterior cingulate cortex (Minoshima et al., 1997) and this includes RSC. An important basis for postulating that NRHypo may play a role in AD is that the disseminated pattern of irreversible neuronal degeneration induced in the adult rat brain by NMDA antagonists (Corso et al., 1997; Wozniak et al., 1998) resembles the pattern of neurofibrillary degeneration in AD. In addition, pyramidal neurons are most vulnerable to NRHypo degeneration and they are also the cell types most vulnerable in AD. Thus, the NRHypo disinhibition model of neurotoxicity could offer a partial explanation for the distribution pattern of neurodegeneration in AD.
Hypofunction of the NMDA receptor system, which is the condition that triggers neurodegeneration in the NRHypo model, is a condition present in the normal aging brain and may be present, to a more exaggerated degree, in the brains of AD patients (Olney et al., 1997). Moreover, it is generally agreed that loss of synaptic complexes is the specific neuropathological change that correlates most closely with cognitive deterioration in AD. The neurotoxicity induced by NMDA antagonists involves the selective deletion of dendritic spines and large numbers of synaptic complexes (Corso et al., 1997; Wozniak et al., 1998) and these changes induced by NMDA antagonists are associated with memory loss in rodents (Wozniak et al., 1996; Brosnan-Watters et al., 1996, 1999). In addition, although hyperphosphorylation of tau protein has been proposed as a mechanism to link neurofibrillary tangle (NFT) formation, only limited headway has been made in understanding the mechanisms that initiate and drive the hyperphosphorylation process. The NRHypo mechanism entails excessive activation of transmitter receptors on the surface of the types of neurons that degenerate in AD and these receptors are linked to second messenger systems which, if hyperactivated, might provide the driving force for a hyperphosphorylation process. Based on these considerations it has been proposed that the NRHypo mechanism acts in concert with amyloid deposition in a multiphase process that results in AD (Olney et al., 1997; Farber et al., 2002c).
Hypofunction of the NMDA receptor system, which is the condition that triggers neurodegeneration in the NRHypo model, is a condition present in the normal aging brain and may be present, to a more exaggerated degree, in the brains of AD patients (Olney et al., 1997). Moreover, it is generally agreed that loss of synaptic complexes is the specific neuropathological change that correlates most closely with cognitive deterioration in AD. The neurotoxicity induced by NMDA antagonists involves the selective deletion of dendritic spines and large numbers of synaptic complexes (Corso et al., 1997; Wozniak et al., 1998) and these changes induced by NMDA antagonists are associated with memory loss in rodents (Wozniak et al., 1996; Brosnan-Watters et al., 1996, 1999). In addition, although hyperphosphorylation of tau protein has been proposed as a mechanism to link neurofibrillary tangle (NFT) formation, only limited headway has been made in understanding the mechanisms that initiate and drive the hyperphosphorylation process. The NRHypo mechanism entails excessive activation of transmitter receptors on the surface of the types of neurons that degenerate in AD and these receptors are linked to second messenger systems which, if hyperactivated, might provide the driving force for a hyperphosphorylation process. Based on these considerations it has been proposed that the NRHypo mechanism acts in concert with amyloid deposition in a multiphase process that results in AD (Olney et al., 1997; Farber et al., 2002c).
Comparison of Medial Cortex in Rat and Monkey
One of the reasons for employing a modification of Brodmann’s original scheme for rodent and primate species is to assure that direct comparisons can be made among species and support a rational process for devising models of human disease. This type of analysis does not presume evolutionary or developmental relationships, although homologies may exist. Rather, it states that areas on the medial surface in all mammalian species undergo a series of architectural transitions and that each area evaluated in this context provides for a direct comparison and areas with the same relative position may be similar among species. Demonstration of similarities between areas in different species and use of a common nomenclature does not imply that two areas with the same designation are exactly equivalent; only that they share enough similarity to explore common mechanisms of disease. Here we consider relations between rat and rhesus monkey.
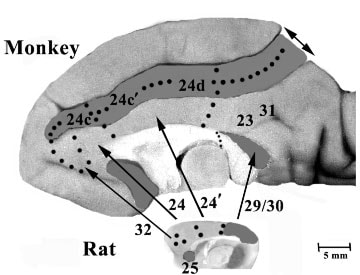
Figure 13 shows the medial surface of both animals with the areas delimited. Areas in monkey cortex that do not appear to have a rodent counterpart are mainly in the cingulate sulcus and include areas 24c, 24c´, 24d as well as the gyral areas 23 and 31. The cingulate sulcus in the monkey was opened so this point could be better appreciated. Although the cingulate motor areas on monkey in areas 24c´ and 24d are not present in rat, there is a part of cingulate cortex in rat that projects to the spinal cord including areas 32 and 24b and it overlaps with AGm (Miller, 1987). Area 24b could be homologous to the rostral cingulate motor area in primates; however, this conclusion suggests that cingulate skeletomotor activity in rat is mediated by pACC, while that in monkey is associated with MCC indicating a very different role of these projections to spinal cord in rat than in monkey.
Success modeling human disease with rodents depends onfinding similarities among the medial surfaces of rat and different primate species. Here cingulate areas in rat and monkey are outlined in photographs at the same magnification. In the monkey the cingulate sulcus was separated (double arrow) to expose the depths of the cingulate sulcus; the splenium of the corpus callosum was warped ventral from the point marked with small dots so the depths of the callosal sulcus can be appreciated. Area 25 in both species is shadowed as are areas 29 and 30 in both species. Although similar cortical regions are smaller in rat, the pericallosal areas in monkey are shown; areas 25, 32, 24a/b, 24a´/b´, and 29a-c, 30. Two regions that do not have counterparts in the rat include monkey areas in the cingulate sulcus (24c, 24c´, 24d) and on the posterior cingulate gyrus (23, 31). The greatest similarity between rat and monkey is in the structure of pericallosal areas.
The massive posterior cingulate gyral surface of primates has no equivalent in rodents, since monkey areas 23a, 23b, and 31 on the gyral surface and area 23c in the caudal cingulate sulcus cannot be identified in rat. RSC in the rodent is comprised of granular area 29 and dysgranular area 30 and this cortex forms the entire PCC in this species. While areas 29 and 30 in the rat are similar to those in primates, these latter areas are actually buried in the callosal sulcus in monkey rather than forming the gyral surface as in rat. The corpus callosum in the monkey in Figure 13 was warped ventrally to expose the depths of the callosal sulcus and demonstrate the retrosplenial areas therein. Areas 23a, 23b and 31, which are on the surface of the posterior cingulate gyrus in monkey and area 23c in the caudal cingulate gyrus together form the PCC in primates and do not appear to have counterparts in the rat. Rose and Woolsey (1948) emphasized this fact by lauding M. Rose’s observations with their following observation: "Area 23 as determined by Brodmann in the rabbit, by Krieg in the rat and by virtually all others except M. Rose, who denied its existence in the rodents, is not likely to exist in any of the loci which have been labeled 23 on rodent cortical maps. M. Rose was obviously right in maintaining that in the rodent’s cortex there is nothing resembling area 23 of carnivores and primates. What appears to be its equivalent area in the rodents has such an outspoken ‘retrosplenial’ appearance that no student of architectonics ever has suggested that it may be equivalent to area 23 in higher forms."
Approximately equivalent areas between the rat and monkey include the following. 1) Area 25 (shaded in both in Figure 13) has a subgenual position. 2) Area 32 is rostral to area 24 in both species. 3) Areas 24a/b and 24a´/b´ have equivalents, although in the rat these areas comprise the entire perigenual and midcingulate regions. 4) Although there is no direct equivalent for the primate sulcal cingulate motor areas 24c´ and 24d in the rat, there are a moderate number of corticospinal projection neurons in rat cingulate cortex as noted above and this supports the notion of a spinal connection, though not from an area that is similar in these species. The different origins of corticospinal projections underscores the less differentiated functions of each rodent cingulate area in contrast to monkey cortex where the corticospinal projections are differentiated into motor areas that are separated from gyral divisions of area 24´. 5) Areas 29a-c appear similar to areas 29l and 29m in monkey and rat area 30 is similar to monkey area 30. Thus, similarities between rat and monkey medial cortices are most prominent in the pericallosal areas.
Even when an area appears to have a similar laminar organization and position in cortical differentiation trends (i.e., periallocortex, proisocortex, isocortex), differences can exist at the connection and cellular levels. At the level of extrinsic connections, it was noted above that corticospinal projections arise from areas in pACC in rat rather than in MCC as in monkey. Also, the primary and secondary visual cortices have major and reciprocal connections with area 29 in rat; however, these do not exist in monkey (Vogt and Pandya, 1987). At the cellular level, even though granular area 29 in rat has a similar counterpart in the monkey, they are not cytologically equivalent. Indeed, the fusiform and extraverted pyramids in rat layers II and III in area 29 have not been observed in monkey (Vogt, 1976; Vogt and Peters, 1981). Finally, at the receptor expression level, presynaptic heteroreceptor organization appears to be different. The presynaptic M2 binding in layers Ia and IV that is so clear in rat has not been observed in monkey (Vogt et al., 1997) where layer I has little dendritic arborization and only weak overall binding for many transmitter receptors due to the presence of a myelin rich fiber tract passing through layer I (taenia tecta).
In spite of the differences between rat and monkey, the essential architecture of pericallosal areas is similar in both species and the rat cortex has significant value as a potential model for certain diseases including neurodegenerative and pharmacological models of psychiatric disease. Given the less differentiated connections, intrinsic organization, and functions of rodent areas, it is unlikely these differences can be overlooked when assessing the mechanisms of cell death and dysfunction. Indeed, rodent models must be considered in the context of these unique morphological and functional properties.
Success modeling human disease with rodents depends onfinding similarities among the medial surfaces of rat and different primate species. Here cingulate areas in rat and monkey are outlined in photographs at the same magnification. In the monkey the cingulate sulcus was separated (double arrow) to expose the depths of the cingulate sulcus; the splenium of the corpus callosum was warped ventral from the point marked with small dots so the depths of the callosal sulcus can be appreciated. Area 25 in both species is shadowed as are areas 29 and 30 in both species. Although similar cortical regions are smaller in rat, the pericallosal areas in monkey are shown; areas 25, 32, 24a/b, 24a´/b´, and 29a-c, 30. Two regions that do not have counterparts in the rat include monkey areas in the cingulate sulcus (24c, 24c´, 24d) and on the posterior cingulate gyrus (23, 31). The greatest similarity between rat and monkey is in the structure of pericallosal areas.
The massive posterior cingulate gyral surface of primates has no equivalent in rodents, since monkey areas 23a, 23b, and 31 on the gyral surface and area 23c in the caudal cingulate sulcus cannot be identified in rat. RSC in the rodent is comprised of granular area 29 and dysgranular area 30 and this cortex forms the entire PCC in this species. While areas 29 and 30 in the rat are similar to those in primates, these latter areas are actually buried in the callosal sulcus in monkey rather than forming the gyral surface as in rat. The corpus callosum in the monkey in Figure 13 was warped ventrally to expose the depths of the callosal sulcus and demonstrate the retrosplenial areas therein. Areas 23a, 23b and 31, which are on the surface of the posterior cingulate gyrus in monkey and area 23c in the caudal cingulate gyrus together form the PCC in primates and do not appear to have counterparts in the rat. Rose and Woolsey (1948) emphasized this fact by lauding M. Rose’s observations with their following observation: "Area 23 as determined by Brodmann in the rabbit, by Krieg in the rat and by virtually all others except M. Rose, who denied its existence in the rodents, is not likely to exist in any of the loci which have been labeled 23 on rodent cortical maps. M. Rose was obviously right in maintaining that in the rodent’s cortex there is nothing resembling area 23 of carnivores and primates. What appears to be its equivalent area in the rodents has such an outspoken ‘retrosplenial’ appearance that no student of architectonics ever has suggested that it may be equivalent to area 23 in higher forms."
Approximately equivalent areas between the rat and monkey include the following. 1) Area 25 (shaded in both in Figure 13) has a subgenual position. 2) Area 32 is rostral to area 24 in both species. 3) Areas 24a/b and 24a´/b´ have equivalents, although in the rat these areas comprise the entire perigenual and midcingulate regions. 4) Although there is no direct equivalent for the primate sulcal cingulate motor areas 24c´ and 24d in the rat, there are a moderate number of corticospinal projection neurons in rat cingulate cortex as noted above and this supports the notion of a spinal connection, though not from an area that is similar in these species. The different origins of corticospinal projections underscores the less differentiated functions of each rodent cingulate area in contrast to monkey cortex where the corticospinal projections are differentiated into motor areas that are separated from gyral divisions of area 24´. 5) Areas 29a-c appear similar to areas 29l and 29m in monkey and rat area 30 is similar to monkey area 30. Thus, similarities between rat and monkey medial cortices are most prominent in the pericallosal areas.
Even when an area appears to have a similar laminar organization and position in cortical differentiation trends (i.e., periallocortex, proisocortex, isocortex), differences can exist at the connection and cellular levels. At the level of extrinsic connections, it was noted above that corticospinal projections arise from areas in pACC in rat rather than in MCC as in monkey. Also, the primary and secondary visual cortices have major and reciprocal connections with area 29 in rat; however, these do not exist in monkey (Vogt and Pandya, 1987). At the cellular level, even though granular area 29 in rat has a similar counterpart in the monkey, they are not cytologically equivalent. Indeed, the fusiform and extraverted pyramids in rat layers II and III in area 29 have not been observed in monkey (Vogt, 1976; Vogt and Peters, 1981). Finally, at the receptor expression level, presynaptic heteroreceptor organization appears to be different. The presynaptic M2 binding in layers Ia and IV that is so clear in rat has not been observed in monkey (Vogt et al., 1997) where layer I has little dendritic arborization and only weak overall binding for many transmitter receptors due to the presence of a myelin rich fiber tract passing through layer I (taenia tecta).
In spite of the differences between rat and monkey, the essential architecture of pericallosal areas is similar in both species and the rat cortex has significant value as a potential model for certain diseases including neurodegenerative and pharmacological models of psychiatric disease. Given the less differentiated connections, intrinsic organization, and functions of rodent areas, it is unlikely these differences can be overlooked when assessing the mechanisms of cell death and dysfunction. Indeed, rodent models must be considered in the context of these unique morphological and functional properties.
Rodent Models of Disease
Morphology at all levels of analysis provides a basis for assessing the extent to which the rat can be used to model primate diseases. If the pericallosal areas play an important role in the onset and/or progression of a disease, the rat is an appropriate choice for model development. However, if the cingulate sulcal or posterior cingulate gyral areas are the primary target, the rodent is not an appropriate animal. Even when pericallosal areas are the primary region of interest, intrinsic differences among rat and primate species could restrict the value of the rat as a model system. For example, although the rat is often used to study the mechanisms of diseases of the basal ganglia, the human has substantially more calretinin-expressing neurons than does the rat and, to the extent that mechanisms of neurodegeneration in movement disorders depend on the calcium-buffering properties of these neurons in human, rat may not be a useful model of these diseases (Wu and Parent, 2000). Indeed, the cytology, connections, and transmitter receptors expressed by afferent axons to area 29 are not the same in rodent and primate and there does not appear to be, for example, heteroreceptor regulation of thalamic afferents in monkey as in rat. Thus, defining animal models for cortical diseases involves determining which areas are similar and the extent to which similar areas have the same organization.
Although the rat has areas 25 and 24a/b that have a similar relative degree of laminar differentiation as in monkey, the rodent has cingulospinal projections that originate from the pACC rather than the cingulate motor areas, which it does not have. Aspects of neurodegeneration in multiple systems atrophy, therefore, may not be ideal candidates for study in rodent cortex. Furthermore, although RSC receives anterior thalamic afferents in rodents and primates, muscarinic, presynaptic heteroreceptors regulate these terminals in rat but not in primate (Vogt et al., 1997). The import of this difference is currently unclear; however, this difference provides a unique opportunity to determine the importance of these receptors in the rodent and what beneficial or detrimental consequences their absence has for primates.
In this chapter we discussed the neurotoxic effects of NMDA antagonists in rodents and how these effects could shed light on certain diseases like schizophrenia and Alzheimer’s disease. An important step that remains is to determine whether a similar neurotoxicity can be induced in non-human primate brain. Obviously, finding similar damage in similar brain regions would be important for advancing our understanding of human physiology and pathophysiology and would testify to the importance of using the NRHypo rodent model to study primate CNS function and disease. However, not finding similar damage in similar brain regions would also provide beneficial information and potentially could be important over the long run for understanding of the primate brain. Finding NRHypo neurotoxicity in non-RSC areas would provide information about the importance of NRHypo neurotoxicity for human biology and may shift interpretations of the psychogenic properties of dissociative anesthetics. Neurotoxicity in other regions in primates might produce the cognitive and behavioral changes (e.g., psychosis) seen with NMDA antagonists.
Ultimately, specifying animal models of human diseases is a dynamic process of refining the cellular and molecular mechanisms of brain structure and function. For example, identifying the distribution of amyloid-_ peptide in early cases of Alzheimer’s disease led to in vitro and in vivo studies of its neurotoxic properties. This led to analysis of its deposition in murine transgenic models that deleted either or both presenilin genes and mutating the amyloid precursor protein gene. Although no one would suggest that behavioral and structural changes in the mouse are equivalent to those in human, the actions of these genes and previous studies in rat and monkey are now serving as a basis for exploring new therapeutic interventions in human clinical trails. Continued progress in understanding the mechanisms of human disease will depend upon hypotheses and mechanistic findings generated via the dynamic interchange among research activities using many mammalian species and a systematized nomenclature serves as a platform for this process.
Although the rat has areas 25 and 24a/b that have a similar relative degree of laminar differentiation as in monkey, the rodent has cingulospinal projections that originate from the pACC rather than the cingulate motor areas, which it does not have. Aspects of neurodegeneration in multiple systems atrophy, therefore, may not be ideal candidates for study in rodent cortex. Furthermore, although RSC receives anterior thalamic afferents in rodents and primates, muscarinic, presynaptic heteroreceptors regulate these terminals in rat but not in primate (Vogt et al., 1997). The import of this difference is currently unclear; however, this difference provides a unique opportunity to determine the importance of these receptors in the rodent and what beneficial or detrimental consequences their absence has for primates.
In this chapter we discussed the neurotoxic effects of NMDA antagonists in rodents and how these effects could shed light on certain diseases like schizophrenia and Alzheimer’s disease. An important step that remains is to determine whether a similar neurotoxicity can be induced in non-human primate brain. Obviously, finding similar damage in similar brain regions would be important for advancing our understanding of human physiology and pathophysiology and would testify to the importance of using the NRHypo rodent model to study primate CNS function and disease. However, not finding similar damage in similar brain regions would also provide beneficial information and potentially could be important over the long run for understanding of the primate brain. Finding NRHypo neurotoxicity in non-RSC areas would provide information about the importance of NRHypo neurotoxicity for human biology and may shift interpretations of the psychogenic properties of dissociative anesthetics. Neurotoxicity in other regions in primates might produce the cognitive and behavioral changes (e.g., psychosis) seen with NMDA antagonists.
Ultimately, specifying animal models of human diseases is a dynamic process of refining the cellular and molecular mechanisms of brain structure and function. For example, identifying the distribution of amyloid-_ peptide in early cases of Alzheimer’s disease led to in vitro and in vivo studies of its neurotoxic properties. This led to analysis of its deposition in murine transgenic models that deleted either or both presenilin genes and mutating the amyloid precursor protein gene. Although no one would suggest that behavioral and structural changes in the mouse are equivalent to those in human, the actions of these genes and previous studies in rat and monkey are now serving as a basis for exploring new therapeutic interventions in human clinical trails. Continued progress in understanding the mechanisms of human disease will depend upon hypotheses and mechanistic findings generated via the dynamic interchange among research activities using many mammalian species and a systematized nomenclature serves as a platform for this process.
Text Abbreviations
|
ac ACC AChE AD AGm A/P AV BDA CYP/IPT DAMGO Glu HRP KA LD MCC NeuN NMDA NRHyper NRHypo OXO-M pACC Post/Ps PrS PZ RSC Sub |
anterior commissure anterior cingulate cortex; comprises perigenual & midcingulate regions acetylcholinesterase anterodorsal thalamic nucleus and Alzheimer’s disease medial agranular motor cortex; also related to M2 and area 6/8 anterior/posterior coordinates anterioventral thalamic nucleus biotinylated dextran amine cyanopindolol/isoproterenol Tyr-D-Ala-Gly-MePhe-Gly-ol glutamate horseradish peroxidase kanic acid laterodorsal thalamic nucleus midcingulate cortex antibody to a neuron-specific neuron nuclear binding protein N-methyl-D-aspartate NMDA receptor hyperfunction NMDA receptor hypofunction oxotremorine M perigenual anterior cingulate cortex postsubiculum presubiculum pirenzepine retrosplenial cortex subiculum |
References
Benes FM (1999) Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biolog Psychiatry, 46, 589-599.
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth.
Brosnan-Watters G, Wozniak DF, Nardi A, Olney JW (1996) Acute behavioral effects of MK-801 in the mouse. Pharmacol, Biochem Behav 53:701-711.
Brosnan-Watters G, Wozniak DF, Nardi A, Olney JW (1999) Parallel recovery of MK-801-induced spatial learning impairment and neuronal injury in male mice. Pharmacol Biochem Behav 62:111-122.
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cog Sci 4:215-222.
Bush G, Vogt BA, Holmes J, Dale, AM, Greve D, Jenike MA, Rosen BR. (2002) Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sc 99:523-528.
Bussey TJ, Muir JL, Everitt BJ, Robbins TW (1997) Triple dissociation of anterior cingulate, posterior cingulate and medial frontal cortices on visual discrimination tasks using a touch screen testing procedure for the rat. Behav Neurosci 111:920-936.
Byatt G, Dalrymple-Alford JC (1996) Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav Neurosci 110:1335-1348.
Casey KL (1999) Forebrain mechanisms of nociception and pain: Analysis through imaging. Proc Natl Acad Sci USA 96:7668-7674.
Castren, E., Da Phena Berzaghi, M., Lindholm, D., & Thoenen, H. (1993). Differential effects of MK-801 on brain-derived neurotrophic factor mRNA levels in different regions of the rat brain. Exper Neurol 122:244-252.
Corso, T. D., Sesma, M. A., Tenkova, T. I., Der, T. C., Wozniak, D. F., Farber, N. B., & Olney, J. W. (1997). Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Res 752:1-14.
Derbyshire SWG (2000) Exploring the pain "neuromatrix." Curr Rev Pain 4:467-477. Donahue RR, LaGraize SC, Fuchs PN. Electrolytic lesion of the anterior cingulate cortex decreases inflammatory but not neuropathic nociceptive behavior in rats. Brain Res 897:131-138.
Donoghue JP, Wise SP (1982) The motor cortex of the rat: Cytoarchitecture and microstimulation mapping. J Comp Neurol 212:76-88.
Dragunow M, Faull RLM (1990) MK-801 induces c-fos protein in thalamic and neocortical neurons of rat brain. Neurosci Lett, 111:39-45.
Dum RP, Strick PL (1993) Cingulate motor areas. In: Vogt BA and Gabriel M (eds) Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 415-441.
Duncan GE, Leipzig JN, Mailman RB, Lieberman JA (1998) Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res, 812:65-75.
Farber NB, Price MT, Labruyere J, Nemnich J, St Peter H, Wozniak DF, Olney JW (1993) Antipsychotic drugs block phencyclidine receptor-mediated neurotoxicity. Biol Psychiatry , 34:119-121.
Farber NB, Foster J, Duhan NL, Olney JW (1995a) a2 adrenergic agonists prevent MK-801 neurotoxicity. Neuropsychopharmacology, 12:347-349.
Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, Olney JW (1995b) Age specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biolog Psychiatry, 38:788-796.
Farber NB, Foster J, Duhan NL, Olney JW (1996) Olanzapine and fluperlapine mimic clozapine in preventing MK-801 neurotoxicity. Schizophrenia Res, 21:33-37.
Farber NB, Hanslick J, Kirby C, McWilliams L, Olney JW (1998) Serotonergic agents that activate 5HT2A receptors prevent NMDA antagonist neurotoxicity. Neuropsychopharmacology, 18:57-62.
Farber NB, Newcomer JW, Olney JW (1999) Glycine Agonists: What can they teach us about schizophrenia? Arch Gen Psychiatry 56:13-17.
Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C (2002) Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol Psychiatry, 7:32-43.
Farber NB, Newcomer JW (2002a) The Role of NMDA Receptor Hypofunction in Idiopathic Psychotic Disorders. In: Child and Early Adolescent Bipolar Disorder. Geller B and Delbello M (eds), New York: Guilford Press, Chap 6, 130-157.
Farber NB, Jiang X-P, Heinkel C, Nemmers B (2002b) Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol Psychiatry, 7:726-733.
Farber NB, Newcomer JW, Olney JW (2002c) Glutamatergic transmission: Therapeutic prospects for schizophrenia and Alzheimer’s Disease. In: Glutamate and GABA Receptors and Transporters: Structure, Function and Pharmacology, Egebjerg, J., Schousboe, A., and Krogsgaard-Larsen, P. (Eds.), London: Taylor and Francis Ltd., Chap 6, pp. 385-406.
Fisk GD, Wyss JM (2000) Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res 859:83-95. J Neurosci 11:1577-1584.
Friedman WJ, Ernfors P, Persson H (1991) Transient and persistent expression of NT-3/BDNF mRNA in the rat brain during postnatal development. J Neurosci 11:1577-1584.
Gonzalo-Ruiz A, Sanz JM, Morte L, Lieberman AR (1997) Glutamate and aspartate immunoreactivity in the reciprocal projections between the anterior thalamic nuclei and the retrosplenial granular cortex in the rat. Brain Res Bull 42:309-327.
Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry, 57, 1061-1069.
Harker KT, Whishaw IQ (2002) Impaired spatial performance in rats with retrosplenial lesions: Importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci 22:1155-1164.
Herkenham M (1976) The connections of the nucleus reunions thalami: Evidence for the direct thalamo-hippocampal pathway in rat. J Comp Neurol 177:589-610.
Hof PR, Lüth H-J, Rogers JH, Celio MR (1993) Calcium-binding proteins define subpopulations of interneurons in cingulate cortex. In: Vogt BA, Gabriel M (eds) Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 181-205.
Horikawa K, Kinjo N, Stanley LC, Powell EW (1988) Topographic organization and collateralization of the projections of the anterior and laterodorsal thalamic nuclei to cingulate areas 24 and 29 in the rat. Neurosci Res 6:31-44.
Hsu M-M, Kung J-C, Shyu B-C (2000) Evoked responses of the anterior cingulate cortex to stimulation of the medial thalamus. Chinese J Physiol 43:81-89.
Hsu M-M, Shyu B-C (1997) Electrophysiological study of the connection between medial thalamus and anterior cingulate cortex in the rat. NeuroReport 8:2701-2707.
Hughes P, Dragunow M, Beilharz E, Lawlor P, Gluckman P (1993) MK801 induces immediate-early gene proteins and BDNF mRNA in rat cerebrocortical neurones. NeuroReport, 4:183-186.
Ibrahim HM, Hogg AJ, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH (2000) Ionotropic Glutamate Receptor Binding and Subunit mRNA Expression in Thalamic Nuclei in Schizophrenia. Am J Psychiatry, 157:1811-1823. Ishimaru M, Fukamauchi F, Olney JW (1995) Halothane prevents MK-801 neurotoxicity in the rat cingulate cortex. Neurosci Lett, 193:1-4.
Jiang X, Dikranian K, Farber NB (2001) Muscimol prevents NMDA antagonist neurotoxicity by acting at gabaergic receptors in the diagonal band and anterior thalamus. Soc Neurosci Abstr 27:973.4.
Johnston MV, McKinney M, Coyle JT (1981) Neocortical cholinergic innervation: A description of extrinsic and intrinsic components in the rat. Exp Brain Res 43:159-172.
Kim SH, Price MT, Olney JW, Farber NB (1999) Excessive cerebrocortical release of acetylcholine induced by NMDA antagonists is reduced by GABAergic and a2-adrenergic agonists. Mol Psychiatry, 4:344-52.
Marini G, Pianca L, Tredici G (1996) Thalamocortical projection from the parafascicular nucleus to layer V pyramidal cells in frontal and cingulate areas of the rat. Neurosci Lett 203:81-84.
Maskati HAA, Zbrozyna AW (1989) Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Autonomic Nervous Sys 28:117-126.
Mesulam M-M (1995) Patterns in behavioral neuroanatomy: Association areas, the limbic system, and hemispheric specialization. In: Principles of Behavioral Neurology. Davis Company: Philadelphia,
Miller MW (1987) The origin of corticospinal projection neurons in rat. Exp Brain Res 67:339-351.
Miller MW, Robertson RT (1993) Development of cingulate cortex: Proteins, neurons, and afferents. In: Vogt BA and Gabriel M (eds), Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp 151-180.
Miller MW, Vogt BA. (1984) Direct connection of rat visual cortex with sensory, motor and association cortices. J Comp Neuro1 226:184-202.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42:85-94.
Morecraft RJ, Van Hoesen GW (1992) Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in cingulate areas 24c and 23c. J Comp Neurol 322:471-489.
Neafsey EJ, Terreberry RR, Hurley KM, Ruit KG, Frysztak RJ (1993) Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, BA Vogt and M Gabriel (eds), Birkhäuser Boston, pp. 206-223.
Olney JW (1990) Excitotoxic amino acids and neuropsychiatric disorders. Ann Rev Pharmacol Toxicol, 30:47-71.
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry, 52:998-1007.
Olney, J. W., Labruyere, J., & Price, M. T. (1989). Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science, 244:1360-1362.
Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA (1991) NMDA antagonist neurotoxicity: mechanism and prevention. Science, 254:1515-1518.
Olney JW, Sesma MA, Wozniak DF (1993) Glutamatergic, Cholinergic, and GABAergic Systems in Posterior Cingulate Cortex. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt, B.A., and Gabriel M. (eds). Birkhauser, Boston, pp. 556-580.
Olney JW, Wozniak DF, Farber NB (1997) Excitotoxic neurodegeneration in Alzheimer Disease; New Hypothesis and New Therapeutic Strategies. Arch Neurol 54(10), 1234-1240.
Paperna T, Malach R (1991) Patterns of sensory intermodality relationships in the cerebral cortex of the rat. J Comp Neurol 308:432-456.
Pastoriza LN, Morrow TJ, Casey KL (1996) Medial frontal cortex lesions selectively attenuate the hot plate response: possible nocifensive apraxia in the rat. Pain 64:11-17.
Paxinos G, Kus L, Ashwell KWS, Watson C (1999) Chemoarchitectonic Atlas of the Rat Forebrain, Academic Press: San Diego.
Paxinos G, Watson C (1986) The Rat Brain in Stereotaxic Coordinates, Sydney: Academic Press. Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30:263-288.
Reep RL, Goodwin GS, Corwin JV (1990) Topographic organization in the corticocortical connections of meadial agranular cortex in rats. J Comp Neurol 294:262-280.
Rieck RW, Carey RG (1985) Organization of the rostral thalamus in the rat: Evidence for connections to layer I of visual cortex. J Comp Neurol 234:137-154.
Rose M (1927) Gyrus limbicus anterior und regio retrosplenialis (Cortex holoprototychus quinquestratificatus). Vergleichende Architektonik bei Tier und Mensch. J Psychol Neurol 35:65-173.
Rose JE, Woolsey CN (1948) Structure of limbic cortex and anterior thalamic nucleiin rabbit and cat. J Comp Neurol 89:279-340.
Sar M, Stumpf WE, Miller RJ, Chang KL, Cuatrecasas P (1978) Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol 182:17-38.
Sato D, Umino A, Kaneda K, Takigawa M, Nishikawa T (1997) Developmental changes in distribution patterns of phencyclidine-induced c-Fos in rat forebrain. Neurosci Lett, 239:21-24.
Sharp FR, Jasper P, Hall J, Noble L, Sagar S M (1991) MK-801 and ketamine induce heat shock protein HSP72 in injured neurons in posterior cingulate and retrosplenial cortex. Ann Neurol, 30:801-809.
Sharp FR, Butman M, Wang S, Koistinaho J, Graham S H, Sagar S M, Noble L, Berger P, Longo FM (1992) Haloperidol prevents induction of the hsp70 heat shock gene in neurons injured by phencyclidine (PCP), MK801, and ketamine. J Neurosci Res, 33:605-616.
Shibata H (1993) Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol 330:533-542.
Shima K, Tanji J (1998) Role of cingulate motor area cells in voluntary movement selection based on reward. Science 282:1335-1338.
Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophsyiol 1992; 68:1720-1731.
Sinnamon HM, Galer BS (1984) Head movements elicited by electrical stimulation of the anteromedial cortex of the rat. Physiol Behav 33:185-190.
Sripanidulchai K, Sripanidulchai B, Wyss JM (1984) The cortical projection of the basolateral amygdaloid nucleus in the rat: A retrograde fluorescent dye study. J Comp Neurol 229:419-431.
Sutherland RJ, Hoesing JM (1993) Posterior cingulate cortex and spatial memory: A microlimnology analysis. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 461-477.
Sutherland RJ, Whishaw IQ, Kolb B (1988) Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci 8:1863-1872.
Taube JS, Muller RU, Ranck Jr JB (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10:420-435.
Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ (1989) Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied with autoradiography and electrophysiology. J Neurosci 9:1764-1773.
Vaccarino AL, Melzack R (1989) Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain 39:213-219.
van Groen T, Vogt BA, Wyss JM (1993) Interconnections between the thalamus and retrosplenial cortex in the rodent brain. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 123-150.
van Groen T, Wyss JM (1995) Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol 358:584-604.
Van Hoesen GW, Morecraft RW, Vogt BA (1993) Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M (eds) Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 249-284.
Vargo JM, Corwin JV, Reep RL (1988) Hemispheric asymmetry in neglect produced by unilateral lesions of dorsomedial prefrontal cortex in rats. Exper Neurol 102:199-209.
Vogt BA (1976) Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. J Comp Neurol 169:63-98.
Vogt BA (1984) Afferent specific localization of muscarinic acetylcholine receptors in cingulate cortex. J Neurosci 4:2191-2199.
Vogt BA (1993) Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, BA Vogt and M Gabriel (eds), Birkhäuser Boston, pp. 19-70.
Vogt BA, Crino PB, Jensen EL (1992) Multiple heteroreceptors on limbic thalamic axons: M2 acetylcholine, serotonin1B, _2-adrenoceptors, _-opioid, and neurotensin. Synapse 10:44-53.
Vogt BA, Derbyshire S, Jones AKP. (1996) Pain processing in four regions of human cingulate cortex localized with coregistered PET and MR imaging. Eur J Neurosci 8:1461-1473.
Vogt BA, Hof PR, Vogt, LJ. (2003) Cingulate Gyrus. In: Paxinos, G. and Mai, JK, eds, The Human Nervous System, second edition, Academic Press, chap 24.
Vogt BA, Miller M. (1983) Cortical connections between rat cingulate cortex and visual, motor and postsubicular cortices. J Comp Neuro1 216:192-210.
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR (1995) Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol 359:490-506.
Vogt BA, Pandya DN. (1987) Cingulate cortex of rhesus monkey. II. Cortical afferents. J Comp Neurol 262:271-289.
Vogt BA, Peters A. (1981) Form and distribution of neurons in rat cingulate cortex: Areas 32, 24 and 29. J Comp Neurol 195:603-625.
Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of rhesus monkey I. Cytoarchitecture and thalamic afferents. J Comp Neurol 1987; 262:256-270.
Vogt BA, Rosene DL, Peters A (1981) Synaptic termination of thalamic and callosal afferents in cingulate cortex of the rat. J Comp Neurol 201:265-283.
Vogt BA, Sikes R W, Vogt LJ. (1993) Anterior cingulate cortex and the medial pain system. In: Vogt BA, Gabriel M, eds, Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston: Birkhäuser, 313-344
Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR (1997) Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer's disease. In: Bloom FE, Björkund A, Hökfelt T, eds, Handbook of Chemical Neuroanatomy. pp. 455-528.
Vogt BA, Vogt, LJ, Perl DP, Hof PR (2001) Cytology of human caudomedial cingulate, retrosplenial and caudal parahippocampal cortices. J Comp Neurol 438:353-376.
Vogt BA, Watanabe H, Grootoonk S, Jones AKP. (1995) Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from PET and MR images. Human Brain Mapping 3:1-12
Vogt BA, Wiley RG, Jensen EL. (1995) Localization of mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of mu regulation. Exp Neurol 135: 83-92.
Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RO, Vogt BA. (2001) Codistribution of mu-opioid receptors and activated G-proteins in rat cingulate cortex. J Pharmacol Exper Ther, 299:840-848.
Vogt LJ, Vogt BA, Sikes RW (1992) Limbic thalamus in rabbit: Architecture, projections to cingulate cortex, and distribution of muscarinic, GABAA, and opioid receptors. J Comp Neurol 319:205-217.
Wiesendanger R, Wiesendanger M (1982) The corticopontine system in the rat: Mapping of corticopontine neurons and the projection pattern. J Comp Neurol 208:215-238.
Woo TU, Whitehead RE, Melchitzky DS, Lewis DA (1998) A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA 95:5341-5346.
Wozniak DF, Brosnan-Watters G, Nardi A, McEwen M, Corso TD, Olney JW, Fix AS. (1996) MK-801 neurotoxicity in male mice: effects and chronic impairment in spatial learning. Brain Res, 707:165-179.
Wozniak DF, Dikranian K, Ishimaru M, Nardi A, Corso TD, Tenkova TI, Olney JW, Fix AS (1998). Disseminated corticolimbic neuronal degeneration induced in rat brain by MK-801: potential relevance to Alzheimer's disease. Neurobiol Disease, 5:305-322.
Wu Y, Parent A (2000) Striatal interneurons expressing calretinin, parvalbumin, oor NADPH-diaphorase: a comparative study in the rat, monkey, and human. Brain Res 863:182-191.
Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R (1996) Morphological and electrophysiological properties of anterior cingulate cortex nociceptive neurons in rats. Brain Res 735:83-92.
Zilles K (1985) The Cortex of the Rat. Springer-Verlag: Berlin.
Zilles K, Wree A (1995) Cortex: areal and laminar structure. In: The Rat Nervous System, G Paxinos (ed.), 2nd Edition, pp 649-685. Academic Press; San Diego.
Zugaro MB, Berthoz A, Wiener SI (2001) Background, but not foreground, spatial cues are taken as references for head direction responses by rat anteroventral thalamus neurons. J Neurosci 21:RC154:1-5.
Benes FM (1999) Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biolog Psychiatry, 46, 589-599.
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth.
Brosnan-Watters G, Wozniak DF, Nardi A, Olney JW (1996) Acute behavioral effects of MK-801 in the mouse. Pharmacol, Biochem Behav 53:701-711.
Brosnan-Watters G, Wozniak DF, Nardi A, Olney JW (1999) Parallel recovery of MK-801-induced spatial learning impairment and neuronal injury in male mice. Pharmacol Biochem Behav 62:111-122.
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cog Sci 4:215-222.
Bush G, Vogt BA, Holmes J, Dale, AM, Greve D, Jenike MA, Rosen BR. (2002) Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sc 99:523-528.
Bussey TJ, Muir JL, Everitt BJ, Robbins TW (1997) Triple dissociation of anterior cingulate, posterior cingulate and medial frontal cortices on visual discrimination tasks using a touch screen testing procedure for the rat. Behav Neurosci 111:920-936.
Byatt G, Dalrymple-Alford JC (1996) Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav Neurosci 110:1335-1348.
Casey KL (1999) Forebrain mechanisms of nociception and pain: Analysis through imaging. Proc Natl Acad Sci USA 96:7668-7674.
Castren, E., Da Phena Berzaghi, M., Lindholm, D., & Thoenen, H. (1993). Differential effects of MK-801 on brain-derived neurotrophic factor mRNA levels in different regions of the rat brain. Exper Neurol 122:244-252.
Corso, T. D., Sesma, M. A., Tenkova, T. I., Der, T. C., Wozniak, D. F., Farber, N. B., & Olney, J. W. (1997). Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Res 752:1-14.
Derbyshire SWG (2000) Exploring the pain "neuromatrix." Curr Rev Pain 4:467-477. Donahue RR, LaGraize SC, Fuchs PN. Electrolytic lesion of the anterior cingulate cortex decreases inflammatory but not neuropathic nociceptive behavior in rats. Brain Res 897:131-138.
Donoghue JP, Wise SP (1982) The motor cortex of the rat: Cytoarchitecture and microstimulation mapping. J Comp Neurol 212:76-88.
Dragunow M, Faull RLM (1990) MK-801 induces c-fos protein in thalamic and neocortical neurons of rat brain. Neurosci Lett, 111:39-45.
Dum RP, Strick PL (1993) Cingulate motor areas. In: Vogt BA and Gabriel M (eds) Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 415-441.
Duncan GE, Leipzig JN, Mailman RB, Lieberman JA (1998) Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res, 812:65-75.
Farber NB, Price MT, Labruyere J, Nemnich J, St Peter H, Wozniak DF, Olney JW (1993) Antipsychotic drugs block phencyclidine receptor-mediated neurotoxicity. Biol Psychiatry , 34:119-121.
Farber NB, Foster J, Duhan NL, Olney JW (1995a) a2 adrenergic agonists prevent MK-801 neurotoxicity. Neuropsychopharmacology, 12:347-349.
Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, Olney JW (1995b) Age specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biolog Psychiatry, 38:788-796.
Farber NB, Foster J, Duhan NL, Olney JW (1996) Olanzapine and fluperlapine mimic clozapine in preventing MK-801 neurotoxicity. Schizophrenia Res, 21:33-37.
Farber NB, Hanslick J, Kirby C, McWilliams L, Olney JW (1998) Serotonergic agents that activate 5HT2A receptors prevent NMDA antagonist neurotoxicity. Neuropsychopharmacology, 18:57-62.
Farber NB, Newcomer JW, Olney JW (1999) Glycine Agonists: What can they teach us about schizophrenia? Arch Gen Psychiatry 56:13-17.
Farber NB, Kim SH, Dikranian K, Jiang XP, Heinkel C (2002) Receptor mechanisms and circuitry underlying NMDA antagonist neurotoxicity. Mol Psychiatry, 7:32-43.
Farber NB, Newcomer JW (2002a) The Role of NMDA Receptor Hypofunction in Idiopathic Psychotic Disorders. In: Child and Early Adolescent Bipolar Disorder. Geller B and Delbello M (eds), New York: Guilford Press, Chap 6, 130-157.
Farber NB, Jiang X-P, Heinkel C, Nemmers B (2002b) Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol Psychiatry, 7:726-733.
Farber NB, Newcomer JW, Olney JW (2002c) Glutamatergic transmission: Therapeutic prospects for schizophrenia and Alzheimer’s Disease. In: Glutamate and GABA Receptors and Transporters: Structure, Function and Pharmacology, Egebjerg, J., Schousboe, A., and Krogsgaard-Larsen, P. (Eds.), London: Taylor and Francis Ltd., Chap 6, pp. 385-406.
Fisk GD, Wyss JM (2000) Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res 859:83-95. J Neurosci 11:1577-1584.
Friedman WJ, Ernfors P, Persson H (1991) Transient and persistent expression of NT-3/BDNF mRNA in the rat brain during postnatal development. J Neurosci 11:1577-1584.
Gonzalo-Ruiz A, Sanz JM, Morte L, Lieberman AR (1997) Glutamate and aspartate immunoreactivity in the reciprocal projections between the anterior thalamic nuclei and the retrosplenial granular cortex in the rat. Brain Res Bull 42:309-327.
Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry, 57, 1061-1069.
Harker KT, Whishaw IQ (2002) Impaired spatial performance in rats with retrosplenial lesions: Importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci 22:1155-1164.
Herkenham M (1976) The connections of the nucleus reunions thalami: Evidence for the direct thalamo-hippocampal pathway in rat. J Comp Neurol 177:589-610.
Hof PR, Lüth H-J, Rogers JH, Celio MR (1993) Calcium-binding proteins define subpopulations of interneurons in cingulate cortex. In: Vogt BA, Gabriel M (eds) Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 181-205.
Horikawa K, Kinjo N, Stanley LC, Powell EW (1988) Topographic organization and collateralization of the projections of the anterior and laterodorsal thalamic nuclei to cingulate areas 24 and 29 in the rat. Neurosci Res 6:31-44.
Hsu M-M, Kung J-C, Shyu B-C (2000) Evoked responses of the anterior cingulate cortex to stimulation of the medial thalamus. Chinese J Physiol 43:81-89.
Hsu M-M, Shyu B-C (1997) Electrophysiological study of the connection between medial thalamus and anterior cingulate cortex in the rat. NeuroReport 8:2701-2707.
Hughes P, Dragunow M, Beilharz E, Lawlor P, Gluckman P (1993) MK801 induces immediate-early gene proteins and BDNF mRNA in rat cerebrocortical neurones. NeuroReport, 4:183-186.
Ibrahim HM, Hogg AJ, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH (2000) Ionotropic Glutamate Receptor Binding and Subunit mRNA Expression in Thalamic Nuclei in Schizophrenia. Am J Psychiatry, 157:1811-1823. Ishimaru M, Fukamauchi F, Olney JW (1995) Halothane prevents MK-801 neurotoxicity in the rat cingulate cortex. Neurosci Lett, 193:1-4.
Jiang X, Dikranian K, Farber NB (2001) Muscimol prevents NMDA antagonist neurotoxicity by acting at gabaergic receptors in the diagonal band and anterior thalamus. Soc Neurosci Abstr 27:973.4.
Johnston MV, McKinney M, Coyle JT (1981) Neocortical cholinergic innervation: A description of extrinsic and intrinsic components in the rat. Exp Brain Res 43:159-172.
Kim SH, Price MT, Olney JW, Farber NB (1999) Excessive cerebrocortical release of acetylcholine induced by NMDA antagonists is reduced by GABAergic and a2-adrenergic agonists. Mol Psychiatry, 4:344-52.
Marini G, Pianca L, Tredici G (1996) Thalamocortical projection from the parafascicular nucleus to layer V pyramidal cells in frontal and cingulate areas of the rat. Neurosci Lett 203:81-84.
Maskati HAA, Zbrozyna AW (1989) Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Autonomic Nervous Sys 28:117-126.
Mesulam M-M (1995) Patterns in behavioral neuroanatomy: Association areas, the limbic system, and hemispheric specialization. In: Principles of Behavioral Neurology. Davis Company: Philadelphia,
Miller MW (1987) The origin of corticospinal projection neurons in rat. Exp Brain Res 67:339-351.
Miller MW, Robertson RT (1993) Development of cingulate cortex: Proteins, neurons, and afferents. In: Vogt BA and Gabriel M (eds), Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp 151-180.
Miller MW, Vogt BA. (1984) Direct connection of rat visual cortex with sensory, motor and association cortices. J Comp Neuro1 226:184-202.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42:85-94.
Morecraft RJ, Van Hoesen GW (1992) Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in cingulate areas 24c and 23c. J Comp Neurol 322:471-489.
Neafsey EJ, Terreberry RR, Hurley KM, Ruit KG, Frysztak RJ (1993) Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, BA Vogt and M Gabriel (eds), Birkhäuser Boston, pp. 206-223.
Olney JW (1990) Excitotoxic amino acids and neuropsychiatric disorders. Ann Rev Pharmacol Toxicol, 30:47-71.
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry, 52:998-1007.
Olney, J. W., Labruyere, J., & Price, M. T. (1989). Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science, 244:1360-1362.
Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA (1991) NMDA antagonist neurotoxicity: mechanism and prevention. Science, 254:1515-1518.
Olney JW, Sesma MA, Wozniak DF (1993) Glutamatergic, Cholinergic, and GABAergic Systems in Posterior Cingulate Cortex. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt, B.A., and Gabriel M. (eds). Birkhauser, Boston, pp. 556-580.
Olney JW, Wozniak DF, Farber NB (1997) Excitotoxic neurodegeneration in Alzheimer Disease; New Hypothesis and New Therapeutic Strategies. Arch Neurol 54(10), 1234-1240.
Paperna T, Malach R (1991) Patterns of sensory intermodality relationships in the cerebral cortex of the rat. J Comp Neurol 308:432-456.
Pastoriza LN, Morrow TJ, Casey KL (1996) Medial frontal cortex lesions selectively attenuate the hot plate response: possible nocifensive apraxia in the rat. Pain 64:11-17.
Paxinos G, Kus L, Ashwell KWS, Watson C (1999) Chemoarchitectonic Atlas of the Rat Forebrain, Academic Press: San Diego.
Paxinos G, Watson C (1986) The Rat Brain in Stereotaxic Coordinates, Sydney: Academic Press. Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30:263-288.
Reep RL, Goodwin GS, Corwin JV (1990) Topographic organization in the corticocortical connections of meadial agranular cortex in rats. J Comp Neurol 294:262-280.
Rieck RW, Carey RG (1985) Organization of the rostral thalamus in the rat: Evidence for connections to layer I of visual cortex. J Comp Neurol 234:137-154.
Rose M (1927) Gyrus limbicus anterior und regio retrosplenialis (Cortex holoprototychus quinquestratificatus). Vergleichende Architektonik bei Tier und Mensch. J Psychol Neurol 35:65-173.
Rose JE, Woolsey CN (1948) Structure of limbic cortex and anterior thalamic nucleiin rabbit and cat. J Comp Neurol 89:279-340.
Sar M, Stumpf WE, Miller RJ, Chang KL, Cuatrecasas P (1978) Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol 182:17-38.
Sato D, Umino A, Kaneda K, Takigawa M, Nishikawa T (1997) Developmental changes in distribution patterns of phencyclidine-induced c-Fos in rat forebrain. Neurosci Lett, 239:21-24.
Sharp FR, Jasper P, Hall J, Noble L, Sagar S M (1991) MK-801 and ketamine induce heat shock protein HSP72 in injured neurons in posterior cingulate and retrosplenial cortex. Ann Neurol, 30:801-809.
Sharp FR, Butman M, Wang S, Koistinaho J, Graham S H, Sagar S M, Noble L, Berger P, Longo FM (1992) Haloperidol prevents induction of the hsp70 heat shock gene in neurons injured by phencyclidine (PCP), MK801, and ketamine. J Neurosci Res, 33:605-616.
Shibata H (1993) Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol 330:533-542.
Shima K, Tanji J (1998) Role of cingulate motor area cells in voluntary movement selection based on reward. Science 282:1335-1338.
Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophsyiol 1992; 68:1720-1731.
Sinnamon HM, Galer BS (1984) Head movements elicited by electrical stimulation of the anteromedial cortex of the rat. Physiol Behav 33:185-190.
Sripanidulchai K, Sripanidulchai B, Wyss JM (1984) The cortical projection of the basolateral amygdaloid nucleus in the rat: A retrograde fluorescent dye study. J Comp Neurol 229:419-431.
Sutherland RJ, Hoesing JM (1993) Posterior cingulate cortex and spatial memory: A microlimnology analysis. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 461-477.
Sutherland RJ, Whishaw IQ, Kolb B (1988) Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci 8:1863-1872.
Taube JS, Muller RU, Ranck Jr JB (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10:420-435.
Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ (1989) Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied with autoradiography and electrophysiology. J Neurosci 9:1764-1773.
Vaccarino AL, Melzack R (1989) Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain 39:213-219.
van Groen T, Vogt BA, Wyss JM (1993) Interconnections between the thalamus and retrosplenial cortex in the rodent brain. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 123-150.
van Groen T, Wyss JM (1995) Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol 358:584-604.
Van Hoesen GW, Morecraft RW, Vogt BA (1993) Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M (eds) Neurobiology of Cingulate Cortex and Limbic Thalamus, Birkhäuser Boston, pp. 249-284.
Vargo JM, Corwin JV, Reep RL (1988) Hemispheric asymmetry in neglect produced by unilateral lesions of dorsomedial prefrontal cortex in rats. Exper Neurol 102:199-209.
Vogt BA (1976) Retrosplenial cortex in the rhesus monkey: A cytoarchitectonic and Golgi study. J Comp Neurol 169:63-98.
Vogt BA (1984) Afferent specific localization of muscarinic acetylcholine receptors in cingulate cortex. J Neurosci 4:2191-2199.
Vogt BA (1993) Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, BA Vogt and M Gabriel (eds), Birkhäuser Boston, pp. 19-70.
Vogt BA, Crino PB, Jensen EL (1992) Multiple heteroreceptors on limbic thalamic axons: M2 acetylcholine, serotonin1B, _2-adrenoceptors, _-opioid, and neurotensin. Synapse 10:44-53.
Vogt BA, Derbyshire S, Jones AKP. (1996) Pain processing in four regions of human cingulate cortex localized with coregistered PET and MR imaging. Eur J Neurosci 8:1461-1473.
Vogt BA, Hof PR, Vogt, LJ. (2003) Cingulate Gyrus. In: Paxinos, G. and Mai, JK, eds, The Human Nervous System, second edition, Academic Press, chap 24.
Vogt BA, Miller M. (1983) Cortical connections between rat cingulate cortex and visual, motor and postsubicular cortices. J Comp Neuro1 216:192-210.
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR (1995) Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol 359:490-506.
Vogt BA, Pandya DN. (1987) Cingulate cortex of rhesus monkey. II. Cortical afferents. J Comp Neurol 262:271-289.
Vogt BA, Peters A. (1981) Form and distribution of neurons in rat cingulate cortex: Areas 32, 24 and 29. J Comp Neurol 195:603-625.
Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of rhesus monkey I. Cytoarchitecture and thalamic afferents. J Comp Neurol 1987; 262:256-270.
Vogt BA, Rosene DL, Peters A (1981) Synaptic termination of thalamic and callosal afferents in cingulate cortex of the rat. J Comp Neurol 201:265-283.
Vogt BA, Sikes R W, Vogt LJ. (1993) Anterior cingulate cortex and the medial pain system. In: Vogt BA, Gabriel M, eds, Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston: Birkhäuser, 313-344
Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR (1997) Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer's disease. In: Bloom FE, Björkund A, Hökfelt T, eds, Handbook of Chemical Neuroanatomy. pp. 455-528.
Vogt BA, Vogt, LJ, Perl DP, Hof PR (2001) Cytology of human caudomedial cingulate, retrosplenial and caudal parahippocampal cortices. J Comp Neurol 438:353-376.
Vogt BA, Watanabe H, Grootoonk S, Jones AKP. (1995) Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from PET and MR images. Human Brain Mapping 3:1-12
Vogt BA, Wiley RG, Jensen EL. (1995) Localization of mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of mu regulation. Exp Neurol 135: 83-92.
Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RO, Vogt BA. (2001) Codistribution of mu-opioid receptors and activated G-proteins in rat cingulate cortex. J Pharmacol Exper Ther, 299:840-848.
Vogt LJ, Vogt BA, Sikes RW (1992) Limbic thalamus in rabbit: Architecture, projections to cingulate cortex, and distribution of muscarinic, GABAA, and opioid receptors. J Comp Neurol 319:205-217.
Wiesendanger R, Wiesendanger M (1982) The corticopontine system in the rat: Mapping of corticopontine neurons and the projection pattern. J Comp Neurol 208:215-238.
Woo TU, Whitehead RE, Melchitzky DS, Lewis DA (1998) A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA 95:5341-5346.
Wozniak DF, Brosnan-Watters G, Nardi A, McEwen M, Corso TD, Olney JW, Fix AS. (1996) MK-801 neurotoxicity in male mice: effects and chronic impairment in spatial learning. Brain Res, 707:165-179.
Wozniak DF, Dikranian K, Ishimaru M, Nardi A, Corso TD, Tenkova TI, Olney JW, Fix AS (1998). Disseminated corticolimbic neuronal degeneration induced in rat brain by MK-801: potential relevance to Alzheimer's disease. Neurobiol Disease, 5:305-322.
Wu Y, Parent A (2000) Striatal interneurons expressing calretinin, parvalbumin, oor NADPH-diaphorase: a comparative study in the rat, monkey, and human. Brain Res 863:182-191.
Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R (1996) Morphological and electrophysiological properties of anterior cingulate cortex nociceptive neurons in rats. Brain Res 735:83-92.
Zilles K (1985) The Cortex of the Rat. Springer-Verlag: Berlin.
Zilles K, Wree A (1995) Cortex: areal and laminar structure. In: The Rat Nervous System, G Paxinos (ed.), 2nd Edition, pp 649-685. Academic Press; San Diego.
Zugaro MB, Berthoz A, Wiener SI (2001) Background, but not foreground, spatial cues are taken as references for head direction responses by rat anteroventral thalamus neurons. J Neurosci 21:RC154:1-5.