Cingulate Gyrus: Regional Morphology, 4 Fundamental Cingulate Divisions
Each part of the cingulate gyrus is designated according to the entire gyral surface and by its particular cytoarchitectural composition. The divisions are not based on a standardized coordinate system because each region cannot be defined with structural images. Rather, each region has specific architectures and this forms the basis for a neurobiological, structure/function model of the cingulate cortex. Traditionally, investigators identify an anterior cingulate region comprised of areas 25, 24 and 32, a posterior cingulate region with areas 23 and 31, and a retrosplenial region composed of areas 29 and 30. This approach failed to account for many observations gleaned over the past two decades and is in the process of major revisions.
In the early 1990s it became clear that anterior cingulate cortex (ACC) is not a single structural entity because amygdala and parietal projections into this region distinguished between its rostral and caudal parts. Figure 1 provides the two monkey cases that were pivotal to differentiating the organization of these two regions. These reconstructions of the medial surface show the distribution of anterogradely labeled axon terminals throughout the cingulate gyrus following tritiated amino acid injections into the amygdala (A.) and inferior and posterior parietal cortex (B.). Although the amygdala has widespread connections throughout the cerebral cortex including primary and secondary visual areas, the primary distribution of this input on the medial surface is to areas 25 and 32 and rostral area 24. There is little input to the midcingulate region and none to posterior cingulate and retrosplenial cortices. In contrast, the parietal projection is massive throughout posterior cingulate cortex and midcingulate cortex but does not appreciably extend into the perigenual part of ACC. In view of the fundamentally different role the amygdala and parietal cortex play in brain function, it is likely their differential projections to ACC represent a watershed boundary in ACC.
In the early 1990s it became clear that anterior cingulate cortex (ACC) is not a single structural entity because amygdala and parietal projections into this region distinguished between its rostral and caudal parts. Figure 1 provides the two monkey cases that were pivotal to differentiating the organization of these two regions. These reconstructions of the medial surface show the distribution of anterogradely labeled axon terminals throughout the cingulate gyrus following tritiated amino acid injections into the amygdala (A.) and inferior and posterior parietal cortex (B.). Although the amygdala has widespread connections throughout the cerebral cortex including primary and secondary visual areas, the primary distribution of this input on the medial surface is to areas 25 and 32 and rostral area 24. There is little input to the midcingulate region and none to posterior cingulate and retrosplenial cortices. In contrast, the parietal projection is massive throughout posterior cingulate cortex and midcingulate cortex but does not appreciably extend into the perigenual part of ACC. In view of the fundamentally different role the amygdala and parietal cortex play in brain function, it is likely their differential projections to ACC represent a watershed boundary in ACC.
Figure 1. Differentiation of perigenual ACC and MCC by amygdala and interior parietal cortex projections in the monkey. Termination of these two major afferents is shown with tritiated amino acid injections that label axon terminals in different parts of cingulate cortex (Vogt and Pandya, 1987). The midcingulate region is identified with two arrows. A. An injection mainly into the lateral and accessory basal nuclei of the amygdala heavily labels axon terminals throughout areas 25 and the rostral part of area 24 or perigenual ACC. B. An injection into inferior parietal cortex labels terminals throughout most of posterior cingulate cortex and there is a substantial termination in MCC including the ventral bank of the cingulate gyrus.
Another pivotal series of observations that led to new views of the organization of ACC were derived from the work of Heiko Braak. His primitive gigantopyramidal field (1976) was in the caudal part of human ACC and it was the first demonstration of a motor area in the cingulatesulcus. This finding was quickly corroborated in the macaque monkey when Biber et al. (1978) described its corticospinal projections and later the details of somatotopically organized projections of cingulate motor cortex to the spinal cord (Dum and Strick, 1993) and motor and limbic cortices were described (Morecraft and Van Hoesen, 1992; Van Hoesen et al., 1993). Heiko Braak (1979b) also detailed the pigmentarchitecture and location of the anterogenual magnoganglionaris area in the rostral sector of ACC and the visceromotor functions of subgenual cortex and their projections to autonomic motor nuclei in the brainstem were identified and reviewed by Neafsey et al. (1993). These cytoarchitecture and connection observations were considered from the overall perspective of cingulate organization and it was proposed that ACC be divided into a perigenual ACC (pACC) and a midcingulate cortex (MCC) (Vogt, 1993). Based on this ACC duality hypothesis, the cytoarchitecture of ACC was assessed in each of these regions in human (Vogt et al., 1995) and the theoretical rationale for making functional distinctions along these lines was considered (Vogt et al., 1997; Vogt and Sikes, 2000).
The rationale for the ACC duality strengthened with numerous functional imaging studies supporting the fact that the rostral and caudal parts of ACC provide substrates for different brain functions. The clearest delineation of these functional units was provided by Whalen et al. (1998) and Bush et al. (1998). In the former study it was shown that the emotional counting Stroop task activated perigenual ACC, while in the latter the counting Stroop activated MCC or their cognitive/motor region. Thus, the border between the rostral and caudal parts of ACC represents important cytoarchitectural, connection, and functional differences. At the present time perigenual ACC is equivalent to anterior ACC and MCC equates to posterior ACC and each region has its own morphology as described in detail below. In the near future the “p” will be removed and the term ACC will be used alone to refer only to the rostral part of ACC. The four fundamental regions of cingulate cortex will not have changed, but their designation will be ACC, MCC, posterior cingulate cortex (PCC), and retrosplenial cortex (RSC).
Another pivotal series of observations that led to new views of the organization of ACC were derived from the work of Heiko Braak. His primitive gigantopyramidal field (1976) was in the caudal part of human ACC and it was the first demonstration of a motor area in the cingulatesulcus. This finding was quickly corroborated in the macaque monkey when Biber et al. (1978) described its corticospinal projections and later the details of somatotopically organized projections of cingulate motor cortex to the spinal cord (Dum and Strick, 1993) and motor and limbic cortices were described (Morecraft and Van Hoesen, 1992; Van Hoesen et al., 1993). Heiko Braak (1979b) also detailed the pigmentarchitecture and location of the anterogenual magnoganglionaris area in the rostral sector of ACC and the visceromotor functions of subgenual cortex and their projections to autonomic motor nuclei in the brainstem were identified and reviewed by Neafsey et al. (1993). These cytoarchitecture and connection observations were considered from the overall perspective of cingulate organization and it was proposed that ACC be divided into a perigenual ACC (pACC) and a midcingulate cortex (MCC) (Vogt, 1993). Based on this ACC duality hypothesis, the cytoarchitecture of ACC was assessed in each of these regions in human (Vogt et al., 1995) and the theoretical rationale for making functional distinctions along these lines was considered (Vogt et al., 1997; Vogt and Sikes, 2000).
The rationale for the ACC duality strengthened with numerous functional imaging studies supporting the fact that the rostral and caudal parts of ACC provide substrates for different brain functions. The clearest delineation of these functional units was provided by Whalen et al. (1998) and Bush et al. (1998). In the former study it was shown that the emotional counting Stroop task activated perigenual ACC, while in the latter the counting Stroop activated MCC or their cognitive/motor region. Thus, the border between the rostral and caudal parts of ACC represents important cytoarchitectural, connection, and functional differences. At the present time perigenual ACC is equivalent to anterior ACC and MCC equates to posterior ACC and each region has its own morphology as described in detail below. In the near future the “p” will be removed and the term ACC will be used alone to refer only to the rostral part of ACC. The four fundamental regions of cingulate cortex will not have changed, but their designation will be ACC, MCC, posterior cingulate cortex (PCC), and retrosplenial cortex (RSC).
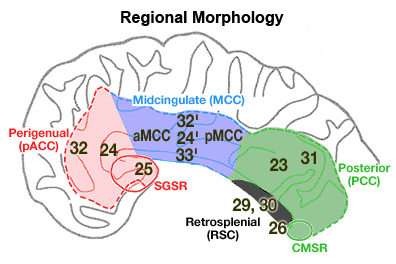
Figure 2. Four region neurobiological model of human cingulate cortex. The model requires close correlations among the topology of cytoarchitectural areas, major afferent connections, regional composition, and functional properties. A. pACC is comprised of areas 25, 33, 24, and 32 and includes a subgenual subregion (SGSR). MCC includes areas 33', 24', 24d, and 32', the PCC is areas 23 and 31 and the caudomedial subregion (CMSR), and the RSC is areas 29 and 30.
Each of the four primary cingulate regions and associated cytoarchitectural areas are shown in Figure 2. The perigenual ACC is that region which surrounds the genu of the corpus callosum and includes areas 25, 33, 32, and 24. Braak (1979b) showed that the region abutting the rostral edge of the genu has a unique architecture and he termed it anterogenual magnogangionaris. The cortex ventral to the genu has also been termed subgenual cortex and is comprised mainly of area 25 and caudoventral extensions of areas 12, 32, and 33. Since this region is likely engaged directly in expression of autonomic states through its projections to the amygdala, nucleus of the solitary tract and the dorsal motor nucleus of the vagus (Neafsey et al., 1993), a separate subregional designation is provided for it: subgenual subregion (SGSR).
The MCC is the middle one-third of the cingulate gyrus and includes the caudal parts of areas 33, 24, and 32 which are designated areas 33', 24', and 32'. Braak’s primitive gigantopyramidal field is a caudal subdivision of area 24' referred to as area 24d by Matelli et al. (1991). The MCC terminates before the inflection of the marginal ramus of the cingulate sulcus. The PCC includes areas 23 and 31 on the posterior cingulate gyrus and the splenial sulci and surrounding cortex. The most caudal and ventral part of these areas forms the caudomedial subregion (CMSR). Although the exact functions of this subregion are not yet known, it does have interesting connections with perigenual ACC (Vogt et al., 1987) and is coactivated with the latter region in auditory-verbal memory tasks (Grasby et al., 1993). Finally, RSC is areas 29 and 30 and they extend around the splenium of the corpus callosum and abut parahippocampal areas at a point just ventral to the ventral edge of the splenium.
The PCC is not equivalent to the posterior cingulate gyrus in primates because the ventral bank of the posterior cingulate gyrus contains RSC. Thus, the posterior cingulate gyrus comprises retrosplenial areas 29 and 30 in the callosal sulcus, PCC on the posterior gyral surface with areas 23a/b and area 31 surrounding the splenial sulci, and area 23c in the depths of the posterior cingulate sulcus, i.e., the ventral part of the marginal ramus of the cingulate sulcus. Posterior cingulate cortex in the human refers primarily to cortex on the gyral surface and that in the caudal part of the cingulate sulcus.
Reconsideration of the structure, connections and functions of cingulate cortex in primates led to a four-region model that replaced the ACC with pACC and MCC. Numerous acute and chronic pain studies show a selective impact on activity in MCC and encourage further study of regional specializations in the structure and functions of cingulate cortex.
Each of the four primary cingulate regions and associated cytoarchitectural areas are shown in Figure 2. The perigenual ACC is that region which surrounds the genu of the corpus callosum and includes areas 25, 33, 32, and 24. Braak (1979b) showed that the region abutting the rostral edge of the genu has a unique architecture and he termed it anterogenual magnogangionaris. The cortex ventral to the genu has also been termed subgenual cortex and is comprised mainly of area 25 and caudoventral extensions of areas 12, 32, and 33. Since this region is likely engaged directly in expression of autonomic states through its projections to the amygdala, nucleus of the solitary tract and the dorsal motor nucleus of the vagus (Neafsey et al., 1993), a separate subregional designation is provided for it: subgenual subregion (SGSR).
The MCC is the middle one-third of the cingulate gyrus and includes the caudal parts of areas 33, 24, and 32 which are designated areas 33', 24', and 32'. Braak’s primitive gigantopyramidal field is a caudal subdivision of area 24' referred to as area 24d by Matelli et al. (1991). The MCC terminates before the inflection of the marginal ramus of the cingulate sulcus. The PCC includes areas 23 and 31 on the posterior cingulate gyrus and the splenial sulci and surrounding cortex. The most caudal and ventral part of these areas forms the caudomedial subregion (CMSR). Although the exact functions of this subregion are not yet known, it does have interesting connections with perigenual ACC (Vogt et al., 1987) and is coactivated with the latter region in auditory-verbal memory tasks (Grasby et al., 1993). Finally, RSC is areas 29 and 30 and they extend around the splenium of the corpus callosum and abut parahippocampal areas at a point just ventral to the ventral edge of the splenium.
The PCC is not equivalent to the posterior cingulate gyrus in primates because the ventral bank of the posterior cingulate gyrus contains RSC. Thus, the posterior cingulate gyrus comprises retrosplenial areas 29 and 30 in the callosal sulcus, PCC on the posterior gyral surface with areas 23a/b and area 31 surrounding the splenial sulci, and area 23c in the depths of the posterior cingulate sulcus, i.e., the ventral part of the marginal ramus of the cingulate sulcus. Posterior cingulate cortex in the human refers primarily to cortex on the gyral surface and that in the caudal part of the cingulate sulcus.
Reconsideration of the structure, connections and functions of cingulate cortex in primates led to a four-region model that replaced the ACC with pACC and MCC. Numerous acute and chronic pain studies show a selective impact on activity in MCC and encourage further study of regional specializations in the structure and functions of cingulate cortex.
References
Biber, M. P., Kneisley, L. W., and LaVail, J. H. (1978). Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp.Neurol. 59, 492-508.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Research 109, 219-233.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. (1979b). Pigment architecture of the human telencephalic cortex. V. Regio anterogenualis. Cell Tissue Res. 204, 441-451.
Bush, G., Whalen, P. J., Rosen, B. R., Jenike, M. A., McInerney, S. C., and Rauch, S. L. (1998). The counting Stroop: An interference task specialized for functional neuroimaging-Validation study with functional MRI. Hum. Brain Mapp. 6, 270-282.
Dum, R. P. and Strick, P. L. (1993). Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 415-441. Birkhäuser, Boston.
Grasby, P. M., Frith, C. D., Friston, K. J., Bench, C., Frackowiak, R. S. J., and Dolan, R. J. (1993). Functional mapping of brain areas implicated in auditory-verbal memory function. Brain 116, 1-20.
Matelli, M., Luppino, G., and Rizzolatti, G. (1991). Architecture of superior and mesial Area 6 and the adjacent cingulate cortex in the macaque monkey. J.Comp.Neurol. 311, 445-462.
Morecraft, R. J. and Van Hoesen, G. W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in Areas 24c and 23c. J.Comp.Neurol. 322, 471-489.
Neafsey, E. J., Terreberry, R. R., Hurley, K. M., Ruit, K. G., and Frysztak, R. J. (1993). Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 207-223. Birkhäuser, Boston.
Vogt, B. A. (1993). Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 19-70. Birkhäuser, Boston.
Vogt, B. A., Nimchinsky, E. A., and Hof, P. R. (1997). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J.Comp.Neurol. 359, 490-506.
Vogt, B. A., Pandya, D. N., and Rosene, D. L. (1987). Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J.Comp.Neurol. 262, 256-270.
Vogt, B. A. and Sikes, R. W. (2000). The medial pain system, cingulate cortex, and parallel processing of nociceptive information. In "Progress In Brain Research" (E. A. Mayer and C. B. Saper, Eds.), Vol. 122 The Biological Basis For Mind Body Interactions, pp. 223-235. Elsevier, Amsterdam.
Whalen, P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A., and Rauch, S. L. (1998). The emotional counting Stroop paradigm: an fMRI probe of the anterior cingulate affective division. Biolog.Psychiatry 44, 1219-1228.
Biber, M. P., Kneisley, L. W., and LaVail, J. H. (1978). Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp.Neurol. 59, 492-508.
Braak, H. (1976). A primitive gigantopyramidal field buried in the depth of the cingulate sulcus of the human brain. Brain Research 109, 219-233.
Braak, H. (1979a). Pigment architecture of the human telencephalic cortex. IV. Regio retrosplenialis. Cell Tissue Res. 204, 431-440.
Braak, H. (1979b). Pigment architecture of the human telencephalic cortex. V. Regio anterogenualis. Cell Tissue Res. 204, 441-451.
Bush, G., Whalen, P. J., Rosen, B. R., Jenike, M. A., McInerney, S. C., and Rauch, S. L. (1998). The counting Stroop: An interference task specialized for functional neuroimaging-Validation study with functional MRI. Hum. Brain Mapp. 6, 270-282.
Dum, R. P. and Strick, P. L. (1993). Cingulate motor areas. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 415-441. Birkhäuser, Boston.
Grasby, P. M., Frith, C. D., Friston, K. J., Bench, C., Frackowiak, R. S. J., and Dolan, R. J. (1993). Functional mapping of brain areas implicated in auditory-verbal memory function. Brain 116, 1-20.
Matelli, M., Luppino, G., and Rizzolatti, G. (1991). Architecture of superior and mesial Area 6 and the adjacent cingulate cortex in the macaque monkey. J.Comp.Neurol. 311, 445-462.
Morecraft, R. J. and Van Hoesen, G. W. (1992). Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in Areas 24c and 23c. J.Comp.Neurol. 322, 471-489.
Neafsey, E. J., Terreberry, R. R., Hurley, K. M., Ruit, K. G., and Frysztak, R. J. (1993). Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 207-223. Birkhäuser, Boston.
Vogt, B. A. (1993). Structural organization of cingulate cortex: Areas, neurons, and somatodendritic transmitter receptors. In "Neurobiology of Cingulate Cortex and Limbic Thalamus" (B. A. Vogt and M. Gabriel, Eds.), pp. 19-70. Birkhäuser, Boston.
Vogt, B. A., Nimchinsky, E. A., and Hof, P. R. (1997). Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J.Comp.Neurol. 359, 490-506.
Vogt, B. A., Pandya, D. N., and Rosene, D. L. (1987). Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J.Comp.Neurol. 262, 256-270.
Vogt, B. A. and Sikes, R. W. (2000). The medial pain system, cingulate cortex, and parallel processing of nociceptive information. In "Progress In Brain Research" (E. A. Mayer and C. B. Saper, Eds.), Vol. 122 The Biological Basis For Mind Body Interactions, pp. 223-235. Elsevier, Amsterdam.
Whalen, P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A., and Rauch, S. L. (1998). The emotional counting Stroop paradigm: an fMRI probe of the anterior cingulate affective division. Biolog.Psychiatry 44, 1219-1228.