Rat Cingulate Cortex & Disease Models (1 of 3)
pathomorphological response to N-methyl-D-aspartate (NMDA) receptor antagonists including MK-801 is made in the context of the cytology and connections of retrosplenial cortex. As a prelude to considering general issues of modeling human disease, the pathomorphological response is related to changes in the schizophrenic and Alzheimer’s diseased brains and regional relations are considered between rat and monkey cingulate cortices. The final section raises issues about using rat cingulate cortex to model human diseases.
Regional Organization
The terms anterior and posterior cingulate cortex are used to designate general regions of cingulate cortex rather than particular cytoarchitectural areas, although there is an underlying morphological basis for this distinction. This difference is based on a granular layer IV in posterior cortex and the lack of a layer IV forming an agranular architecture in anterior cortex. This simple dichotomy, however, leads to confusion on three fronts. First, functional imaging studies in human medial cortex suggest there are many functional specializations in the cingulate gyrus that cannot be accommodated by a simple dichotomy in cingulate structure. Second, ACC is not a single entity but two based on structural and functional assessments as noted above (pACC and MCC) and they are present in rodent. Third, posterior cingulate cortex (PCC) in rat is not equivalent to the same general region in primates. In rodents it is comprised entirely of retrosplenial areas 29 and 30 and there is no cingulate gyrus because a delimiting cingulate sulcus is not present. In primates, retrosplenial areas 29 and 30 are in the callosal sulcus on the ventral bank of the cingulate gyrus, while the exposed gyral surface is comprised of areas 23 and 31. PCC is not interchangeable in rodents and primates.
The duality of rat ACC was first proposed to account for structural, connection and limited functional observations (Vogt, 1993). The pACC receives most prominent amygdala input (Sripanidkulchai et al., 1984) and MCC projects strongly to the pontine nuclei, while pACC does not (Wiesendanger and Wiesendanger, 1982). The MCC transiently expresses oxytocin receptors and neurotrophin-3 (Tribollet et al., 1989, Friedman et al., 1991) and adults have an opioid architecture that differs from pACC (discussed below). Finally, extensive human imaging studies have defined a border between pACC and MCC (Bush et al., 2000). These fundamental differences in connections, transmitters and functions require re-definition of rat cingulate cytology that is compatible with evolving understanding of primate cortex including that in human brain.
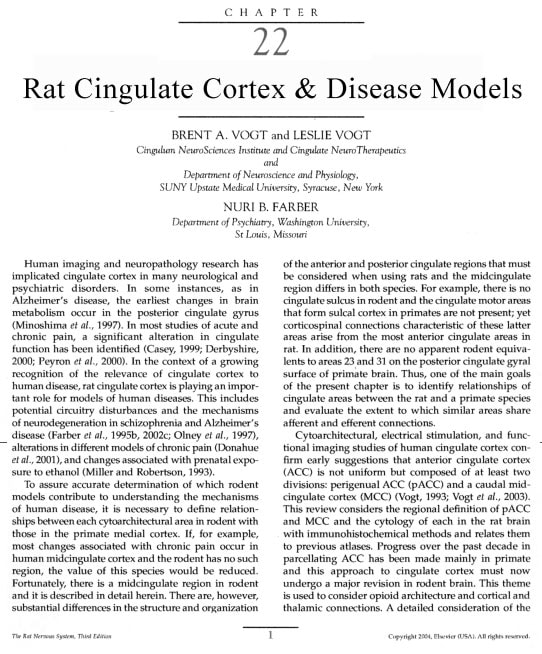
The three regions of rat cingulate cortex are shown in Figure 1 in a modification of our original rat map (Vogt and Peters, 1981). This revision includes localization of each region (pACC; MCC; and retrosplenial cortex, RSC), shows the distribution of each area and subarea, redefines dysgranular area 29d as area 30 to draw a comparison with an area of the same cortical moiety in monkey, and provides an approximation of the anterior/posterior coordinates for each major region and area in relation to the Paxinos and Watson atlas (1986). The pACC is comprised of areas 25, 32, and 24a/b. Realizing that MCC is a posterior division of area 24, it is designated area 24´ and includes areas 24a´ and 24b´. A similar strategy has been applied to the divisions of "Cg" by Zilles and Wree (1995). Although PCC is equivalent to RSC in rat, confusion is generated by the use of PCC for rat cortex because primates have a massive expanse of posterior cingulate areas 23 and 31 that are not present in rodents. Since RSC is equivalent to PCC in rat but not primate PCC, the regional designation of RSC is used here for the posterior region in rat brain. Finally, Chapter 23 provides a comparison of the nomenclatures used for each cingulate and retrosplenial area in rat.
The duality of rat ACC was first proposed to account for structural, connection and limited functional observations (Vogt, 1993). The pACC receives most prominent amygdala input (Sripanidkulchai et al., 1984) and MCC projects strongly to the pontine nuclei, while pACC does not (Wiesendanger and Wiesendanger, 1982). The MCC transiently expresses oxytocin receptors and neurotrophin-3 (Tribollet et al., 1989, Friedman et al., 1991) and adults have an opioid architecture that differs from pACC (discussed below). Finally, extensive human imaging studies have defined a border between pACC and MCC (Bush et al., 2000). These fundamental differences in connections, transmitters and functions require re-definition of rat cingulate cytology that is compatible with evolving understanding of primate cortex including that in human brain.
The three regions of rat cingulate cortex are shown in Figure 1 in a modification of our original rat map (Vogt and Peters, 1981). This revision includes localization of each region (pACC; MCC; and retrosplenial cortex, RSC), shows the distribution of each area and subarea, redefines dysgranular area 29d as area 30 to draw a comparison with an area of the same cortical moiety in monkey, and provides an approximation of the anterior/posterior coordinates for each major region and area in relation to the Paxinos and Watson atlas (1986). The pACC is comprised of areas 25, 32, and 24a/b. Realizing that MCC is a posterior division of area 24, it is designated area 24´ and includes areas 24a´ and 24b´. A similar strategy has been applied to the divisions of "Cg" by Zilles and Wree (1995). Although PCC is equivalent to RSC in rat, confusion is generated by the use of PCC for rat cortex because primates have a massive expanse of posterior cingulate areas 23 and 31 that are not present in rodents. Since RSC is equivalent to PCC in rat but not primate PCC, the regional designation of RSC is used here for the posterior region in rat brain. Finally, Chapter 23 provides a comparison of the nomenclatures used for each cingulate and retrosplenial area in rat.
Figure 1 Overview of the rat medial cortex including the cingulate areas within the heavy line and adjacent areas 10, AGm, and 18b. The three regional designations identify approximate anterior-posterior levels (A/P) of the pACC, MCC, and RSC regions. The pACC includes areas 25, 32, and 24, MCC is comprised of area 24´, and RSC is comprised of areas 29 and 30. The A/P coordinates are from Paxinos and Watson (1986).
Is "Infra"limbic Area IL Ventral to Limbic Cortex?
Although the nomenclature of Rose (1927) has been used frequently for studies of rodent cingulate cortex, it raises important questions about the organization of this region. It suggests an "infra"limbic area, which is similar to Brodmann’s area 25, lies below limbic cortex, rather than being limbic cortex itself. Although there have also been proposals that all cingulate cortex is "para"limbic rather than limbic (Mesulam, 1995), to characterize part of ACC as "infra" or "para" requires a definition of what constitutes limbic cortex. For the designation of "para"limbic, a cortex needs to abut the indusium griseum; a structure that is less than 0.01% the size of the human cingulate gyrus. Indeed, referring to cingulate cortex as "para"limbic says nothing about what the cingulate cortex is or what it does. We prefer a functional definition for a limbic area that includes any cortex with a specific role in regulating autonomic responses, dense projections to the hypothalamus, and subserving emotion (positive or negative internal states and associated memories). To the extent the posterior hippocampus and indusium griseum are involved in general short-term memory formation including emotional memories, they do not have a specific role in emotion and are not limbic by this definition. This functional definition identifies area 25 as a major limbic area. It has been termed visceromotor cortex and has projections to the nucleus of the solitary tract and dorsal motor nucleus of the vagus (Neafsey et al., 1993) and may mediate autonomic activity through the amygdala (Fisk and Wyss, 2000). Direct modulation of autonomic activity assures that area 25 is limbic cortex rather than "infra"limbic.
If one accepts the functional definition for a limbic structure, it becomes clear the posterior hippocampal, posterior cingulate and retrosplenial cortices are not limbic because they are not known to have a specific role in regulating emotion and associated autonomic responses. The confusion over these areas as being limbic derives from a century-old notion that cortices with a "simple" laminar architecture are limbic without consideration of their roles in brain function. An irony of this view is that even on this early anatomical criterion, area 25 is more characteristic of a limbic cortex than area 24 because area 25 has less differentiated layers than does area 24. To demonstrate this point, we begin the cytological analysis of cingulate cortex below with a side-by-side comparison of these two areas. Thus, area 25 is not "infra" (below) or "para" (adjacent) to limbic cortex but, rather, it is limbic in more direct ways than area 24. Furthermore, the evidence from human studies is very compelling that area 25 has a direct role in regulating autonomic functions and generating emotional states (Vogt et al., 2003). Thus, area 25 is limbic cortex and use of the IL concept leads to misuse of the term limbic.
If one accepts the functional definition for a limbic structure, it becomes clear the posterior hippocampal, posterior cingulate and retrosplenial cortices are not limbic because they are not known to have a specific role in regulating emotion and associated autonomic responses. The confusion over these areas as being limbic derives from a century-old notion that cortices with a "simple" laminar architecture are limbic without consideration of their roles in brain function. An irony of this view is that even on this early anatomical criterion, area 25 is more characteristic of a limbic cortex than area 24 because area 25 has less differentiated layers than does area 24. To demonstrate this point, we begin the cytological analysis of cingulate cortex below with a side-by-side comparison of these two areas. Thus, area 25 is not "infra" (below) or "para" (adjacent) to limbic cortex but, rather, it is limbic in more direct ways than area 24. Furthermore, the evidence from human studies is very compelling that area 25 has a direct role in regulating autonomic functions and generating emotional states (Vogt et al., 2003). Thus, area 25 is limbic cortex and use of the IL concept leads to misuse of the term limbic.
Cytology of Limbic Area 25
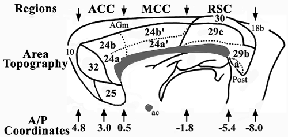
Area 25 is one of the least differentiated cingulate areas in rat; the other being area 29a in RSC. An early stage of differentiation is usually associated with large pyramidal somata, high neuron densities in layers V and II, and poor laminar differentiation; i.e., laminar subdivisions are difficult to detect. Figure 2 shows examples of all cingulate areas with an antibody to neuron-specific nuclear binding protein (NeuN) and at four levels there are silver-stained sections from another animal to show the distribution of axons in similar areas. Since the NeuN antibody does not label glial or vascular elements, it provides a clear picture of laminar architecture. Levels A and B in this figure can be used to evaluate areas 25 and 24a, respectively, and there are higher magnification photographs for comparison of the layers. Area 25 has much larger neuronal somata throughout all layers than does area 24a. Area 25 has a very broad layer II and a poorly differentiated layer III, layer V is uniform and relatively thick and a layer VI is thin and hard to detect. In contrast, area 24a is generally thicker and has smaller neuronal somata than area 25, it has a thinner layer II, broader layers III, V and VI, and many more parvalbumin-expressing neurons than does area 25 (i.e., areas Cg and IL; Paxinos et al., 1999). Also, the superficial part of layer V has larger neurons and this further enhances laminar differentiation. Thus, on anatomical grounds, area 25 is the least differentiated area of ACC
Figure 2. Structure of cingulate cortex at different rostrocaudal levels. A-E each have a NeuN-stained, coronal section to show the laminar distribution of neurons unconfounded by co-staining of glial and vascular elements and B-E have an additional silver pyridine-stained section to show the distribution of large caliber axons. Of particular note are the following: area 25 has a very poorly differentiated laminar architecture when compared to area 24a (higher magnification photographs at bottom), area 24 is not uniform and the area 24/24´ dichotomy is supported, and the internal plexiform layer of area 29c is pronounced (D, silver stain).
Modified Brodmann Nomenclature
Most functional studies of human cortex employ the Brodmann (1909) nomenclature to designate sites of activity and none use the Rose nomenclature that is so frequently used in rodent studies. Therefore, nomenclatures often used in rodents have not been directly applied to human medial cortex. The resulting paradox is that findings in rat cortex cannot be directly related to the structure and functions of primate cortex and a disconnect has developed between research in rat brain and this makes modeling human diseases difficult in terms of an extensive and growing body of human research in normal and pathological states.
Taking from our own experience, how does one relate the distribution of neurodegeneration in rat cortical areas following MK-801 exposure to the pattern of cell death in human Alzheimer’s disease cases, if the former is to be considered a model of the latter? If there is no similarity among areas in rat and human brains, it will be difficult to model human diseases in the rat. Indeed, although no area in rat can be truly equivalent to that in human, one can characterize areas that share structural features. The hippocampus, for example, is often analyzed in transgenic mouse models of Alzheimer’s disease and it is suggested that changes in both species are related. If this strategy can be used in the hippocampus, why should it not be applied to medial cortex? Consider area 29, for example. Area 29 in both species lies between allocortical and isocortical regions and contains poorly differentiated granular layers. This does not mean, however, that they are the same. Area 29 in rat receives direct inputs from primary and secondary visual cortices (i.e., areas 17 and 18, respectively) and this is not the case in monkey (Vogt and Miller, 1983; Vogt and Pandya, 1987). The rat granular layer has both fusiform and extraverted pyramids, while the monkey has neither of these types of neurons (Vogt and Peters, 1981; Vogt, 1976). Thus, rat area 29 is similar in ways to that in monkey area 29 and there may be instances when neurodegeneration in rat area 29 may be a model of cell death in some human diseases. This does not mean, however, they are equivalent.
When Brodmann’s scheme was originally modified for rat cingulate cortex (Vogt and Peters, 1981), it was done in relation to work in monkey (Vogt et al., 1987) and it was eventually related directly to human cortex (Vogt et al., 1995). Each cingulate area in rodent can be evaluated for counterparts in primate cortex determined from relative cortical differentiation, phenotypic expression of particular peptides, and common connections and functions. Although this undertaking is an active area of research, an example of this type of undertaking is provided below under "Comparison of Medial Cortex in Rat and Monkey."
Taking from our own experience, how does one relate the distribution of neurodegeneration in rat cortical areas following MK-801 exposure to the pattern of cell death in human Alzheimer’s disease cases, if the former is to be considered a model of the latter? If there is no similarity among areas in rat and human brains, it will be difficult to model human diseases in the rat. Indeed, although no area in rat can be truly equivalent to that in human, one can characterize areas that share structural features. The hippocampus, for example, is often analyzed in transgenic mouse models of Alzheimer’s disease and it is suggested that changes in both species are related. If this strategy can be used in the hippocampus, why should it not be applied to medial cortex? Consider area 29, for example. Area 29 in both species lies between allocortical and isocortical regions and contains poorly differentiated granular layers. This does not mean, however, that they are the same. Area 29 in rat receives direct inputs from primary and secondary visual cortices (i.e., areas 17 and 18, respectively) and this is not the case in monkey (Vogt and Miller, 1983; Vogt and Pandya, 1987). The rat granular layer has both fusiform and extraverted pyramids, while the monkey has neither of these types of neurons (Vogt and Peters, 1981; Vogt, 1976). Thus, rat area 29 is similar in ways to that in monkey area 29 and there may be instances when neurodegeneration in rat area 29 may be a model of cell death in some human diseases. This does not mean, however, they are equivalent.
When Brodmann’s scheme was originally modified for rat cingulate cortex (Vogt and Peters, 1981), it was done in relation to work in monkey (Vogt et al., 1987) and it was eventually related directly to human cortex (Vogt et al., 1995). Each cingulate area in rodent can be evaluated for counterparts in primate cortex determined from relative cortical differentiation, phenotypic expression of particular peptides, and common connections and functions. Although this undertaking is an active area of research, an example of this type of undertaking is provided below under "Comparison of Medial Cortex in Rat and Monkey."
Cytology of the Perigenual Anterior and Midcingulate Regions
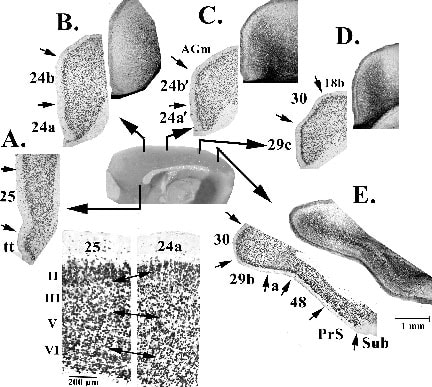
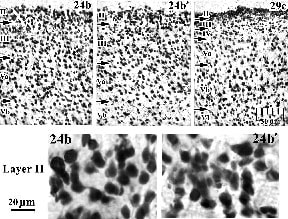
One of the most important current considerations is the differentiation of ACC into a pACC and MCC regions at the cytological, connection, and transmitter system levels of organization. Area 24´ designates the posterior division of area 24 or MCC and this emphasizes that area 24´ is an agranular cortex and not simply a narrow "transitional" region with a mixture of both anterior and posterior cortical features. Differentiation of areas 24 and 24´ is shown in Figure 2 with NeuN immunoreactivity for neurons and differentiated, silver pyridine sections for large axons. The dorsal part of pACC is in B. and MCC is in C. for both stains, respectively. These macrophotographs show that layer II in area 24 is thick relative to that in area 24´ and that the thickness from pia to white matter is greater in area 24. The detailed differences in laminar architecture between areas 24 and 24´ are in Figure 3 for the "b" subdivisions. Although there are occasional large neurons in layer II of area 24´, they are generally smaller and more numerous than in area 24. Comparison of both divisions to area 29c in the same figure suggests there is a transition throughout cingulate cortex that culminates in small and densely packed neurons in the external layers. In addition to this trend that is most pronounced in layer II, layer III is thicker in area 24b´ than area 24 and layer Va in area 24 is more neuron dense than in 24´. In silver-stained tissue an axonal plexus is in layer I of both divisions of area 24, it is thicker and more neuron dense in area 24´ than area 24. Also, the relative differentiation of the a/b subdivisions is more pronounced in area 24´. The layer I plexus in area 24a´ extends into layers II and III, while in area 24b´ the plexus clearing is more pronounced in layer II and top of layer III. Finally, there is a substantially greater density of large axons in layers V and VI of area 24´ than is the case for area 24 and axonal staining confirms cytological observations of areas pACC and MCC.
Figure 3. Differential architectures of areas 24b and 24b´ and their comparison to area 29c. In addition to the generally higher density of neurons in area 24 than area 24´, the neurons in layer II of area 24b are larger than those in area 24b´. Although some size differences may also exist in layers III and V, they will require a quantitative analysis.
Cytology of Retrosplenial Cortex
Retrosplenial cortex forms approximately the posterior one-third of the medial surface. Beginning with the most ventral area 29a, each subdivision contributes to a progressive elaboration that focuses mainly on the granular layers, but also involves differentiation of layers V and VI. Area 29a abuts the postsubiculum (area 48) as shown in Figure 2.
The transition to area 48 is characterized by smaller somata in area 29a. The latter area has an external pyramidal layer composed of a very thin layer II and a layer III/IV (Fig. 4), where layer IV is hardly perceptible. Nissl stains with their co-staining of glial elements originally suggested this is a homogeneous granular layer (Vogt and Peters, 1981). The deep pyramidal layer is quite homogeneous, although a thin layer VI of small neurons can be detected. Area 29b, in contrast, has a very dense and thick layer II of usually 3-5 neuronal somata depth, dispersed and granular layer III, and a thin layer IV that is neuron sparse. It might be argued a layer IV is not present; however, areas 29b and 29c receive a thin layer of thalamic terminals in this layer and a silver-stained axonal plexus can also be detected therein (Fig. 2). Thus, this distinction is based on more than cell structure considerations. Layer V has large typical pyramids and there is a size differential with deeper neurons being larger. Layer VI, though thin, is quite clear.
The transition to area 48 is characterized by smaller somata in area 29a. The latter area has an external pyramidal layer composed of a very thin layer II and a layer III/IV (Fig. 4), where layer IV is hardly perceptible. Nissl stains with their co-staining of glial elements originally suggested this is a homogeneous granular layer (Vogt and Peters, 1981). The deep pyramidal layer is quite homogeneous, although a thin layer VI of small neurons can be detected. Area 29b, in contrast, has a very dense and thick layer II of usually 3-5 neuronal somata depth, dispersed and granular layer III, and a thin layer IV that is neuron sparse. It might be argued a layer IV is not present; however, areas 29b and 29c receive a thin layer of thalamic terminals in this layer and a silver-stained axonal plexus can also be detected therein (Fig. 2). Thus, this distinction is based on more than cell structure considerations. Layer V has large typical pyramids and there is a size differential with deeper neurons being larger. Layer VI, though thin, is quite clear.
Figure 4. Laminar architectures in the four divisions of area 29 with NeuN immunohistochemistry. The progressive elaboration of laminar differences and cortical thickness is apparent beginning with the least differentiated area 29a. Although there is some differentiation of the external layers into layers II and III/IV, the neurons in layer II are larger and less dense in area 29a than they are in area 29c. In addition to the highest level of external pyramidal layer differentiation in area 29c, layer Vb has the largest neurons in layer V and there appear to be two divisions of layer VI. Finally, dysgranular area 30 has larger layers II and III pyramidal neurons and layer IV has clumps of small neurons.
Area 29c cytology is shown in detail in Figures 3 and 4. It has the most differentiated granular layers and, overall, the neurons tend to be smaller in diameter than areas 29a and b. The predominant neuron types are the fusiform and star pyramids (Vogt and Peters, 1981). Examples of these small neurons are also shown later in Figure 8 from a preparation in which they were backfilled from contralateral cortex. The apical dendrites of these neurons form bundles as do their apical tufts in layer Ia and they are sensitive to aging processes and form fewer branches than in young adult animals. One consequence of this aging process could be impaired visuospatial learning that is mediated in part by area 29 (van Groen et al., 1993). Other examples of the apical tuft distributions in layer Ia for fusiform and star pyramids are shown later in Figure 10 in the context of the pathomorphological response to NMDA receptor antagonists.
In the deeper layers of area 29c, differentiation of layer V is very pronounced (Fig. 3) where layer Va is formed by medium-sized and more diffusely packed pyramidal neurons than is layer Vb. This layer V organization is characteristic of "motor" cortices where the large layer V corticospinal projection neurons are mainly in deep layer V and may suggest the important role of this region in behavioral performance in addition to acquisition. Finally, Layer VI is quite thick and supports a heavy interaction of area 29c with the anterior thalamic nuclei.
Dorsal to area 29c lies Brodmann’s area 29d, which Rose (1927) referred to as agranular and is often termed "RSA" (see also Chapter 23). Although an equivalent area in primate is termed area 30, it is not agranular but rather dysgranular (Vogt et al., 2001). Since the rat and monkey share this dysgranular cortex, the rat area 29d is now termed area 30 for consistency between species and to recognize its fundamentally different architecture from that of the granular parts of area 29. In the rat, the posterior cingulate region undergoes almost continual transition as shown in Figures 2D/E and 4. Layers II-III are comprised of larger neurons that are progressively more dispersed in the dorsal cortex. The silver-stained axons show a disappearance of the layer IV plexus and a progressive widening of these layers and reduction in the overall density of large diameter axons. At higher magnification (Fig. 4), all of these features are apparent and it can be seen there is a small and dysgranular layer IV. Thus, area 30 is not truly agranular as is area 24. Indeed, in rat, even motor cortex can have a layer IV and is not truly agranular (Donoghue and Wise, 1982). It is for these reasons that early anatomists may have overlooked the dysgranular nature of area 30. Finally, the presence of layer IV is confirmed by a very dense projection of the laterodorsal nucleus to layer IV of this area as discussed below.
Area 29c cytology is shown in detail in Figures 3 and 4. It has the most differentiated granular layers and, overall, the neurons tend to be smaller in diameter than areas 29a and b. The predominant neuron types are the fusiform and star pyramids (Vogt and Peters, 1981). Examples of these small neurons are also shown later in Figure 8 from a preparation in which they were backfilled from contralateral cortex. The apical dendrites of these neurons form bundles as do their apical tufts in layer Ia and they are sensitive to aging processes and form fewer branches than in young adult animals. One consequence of this aging process could be impaired visuospatial learning that is mediated in part by area 29 (van Groen et al., 1993). Other examples of the apical tuft distributions in layer Ia for fusiform and star pyramids are shown later in Figure 10 in the context of the pathomorphological response to NMDA receptor antagonists.
In the deeper layers of area 29c, differentiation of layer V is very pronounced (Fig. 3) where layer Va is formed by medium-sized and more diffusely packed pyramidal neurons than is layer Vb. This layer V organization is characteristic of "motor" cortices where the large layer V corticospinal projection neurons are mainly in deep layer V and may suggest the important role of this region in behavioral performance in addition to acquisition. Finally, Layer VI is quite thick and supports a heavy interaction of area 29c with the anterior thalamic nuclei.
Dorsal to area 29c lies Brodmann’s area 29d, which Rose (1927) referred to as agranular and is often termed "RSA" (see also Chapter 23). Although an equivalent area in primate is termed area 30, it is not agranular but rather dysgranular (Vogt et al., 2001). Since the rat and monkey share this dysgranular cortex, the rat area 29d is now termed area 30 for consistency between species and to recognize its fundamentally different architecture from that of the granular parts of area 29. In the rat, the posterior cingulate region undergoes almost continual transition as shown in Figures 2D/E and 4. Layers II-III are comprised of larger neurons that are progressively more dispersed in the dorsal cortex. The silver-stained axons show a disappearance of the layer IV plexus and a progressive widening of these layers and reduction in the overall density of large diameter axons. At higher magnification (Fig. 4), all of these features are apparent and it can be seen there is a small and dysgranular layer IV. Thus, area 30 is not truly agranular as is area 24. Indeed, in rat, even motor cortex can have a layer IV and is not truly agranular (Donoghue and Wise, 1982). It is for these reasons that early anatomists may have overlooked the dysgranular nature of area 30. Finally, the presence of layer IV is confirmed by a very dense projection of the laterodorsal nucleus to layer IV of this area as discussed below.
Opioid Architecture; Regional Differences and Neuronal Expression Patterns
It has long been known that rat anteromedial cortex has the highest density of opioid receptors and enkephalin expressing interneurons (Sar et al., 1978). Highest binding in human brain is in pACC with lesser amounts in MCC, and least in PCC including RSC (Vogt et al., 1995). Experimental studies in rat have provided much information about the organization of cortical opioid systems including its regional differentiation in cingulate cortex. In the context of the motor functions of ACC discussed below, including those associated with pain processing, it is important to consider the distribution of opioid receptors and how their activation might modulate motor functions.
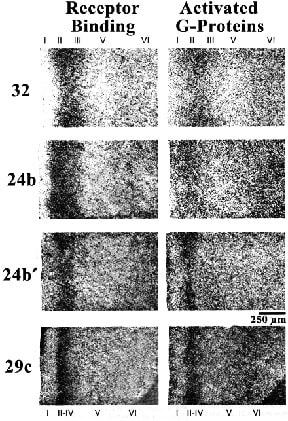
Expression of the µ-opioid receptor agonist Tyr-D-Ala-Gly-MePhe-Gly-ol (DAMGO) has been used for receptor binding and DAMGO-stimulated GTPgS stimulation with autoradiography to assess the overall composition of opioid circuits and provides insight into differentiation of pACC and MCC (L. Vogt et al., 2001). This study showed highest binding in area 32, intermediate amounts in areas 24 and 24´ and least in area 29 (Fig. 5), thus mimicking the regional differentiation observed in human brain. While area 24´ shared a similar laminar pattern of µ-opioid binding with area 24 (highest in layer V and moderate in layers I and VI), area 24´ shared a similar pattern of GTPgS stimulation with area 29 (moderate to low levels in layers I and V-VI). A correlation analysis of DAMGO binding and DAMGO-stimulated GTPgS activity confirmed that area 24´ has a unique opioid architecture from that of either areas 24 or 29. This confirms the regional differentiation of rodent cingulate cortex into pACC, MCC, and PCC.
Early studies reported a layer I concentration of µ-opioid receptors in ACC; however, experimental studies were needed to identify those components of the cortical neuropil that expressed the receptors in their dendrites and axonal terminals. Undercut lesions that remove all afferent axons, and therefore presynaptic receptors, show that about 30% layer II-VI binding is lost in area 24 and 50% is lost in layers I and V in area 29 (LJ Vogt et al., 2001; Vogt et al., 1995). The remaining binding following undercut lesions is expressed by the soma/dendritic membranes and possibly some glia. The question remains, however, which afferent axonal systems express µ-opioid receptors. There are at least two such sources. First, the locus coeruleus synthesizes µ-receptors and they are transported to presynaptic terminals in cingulate cortex. This was shown using the neurotoxin saporin conjugated to dopamine-b hydroxylase to kill neurons in the locus coeruleus followed by autoradiographic assay of binding and G-protein stimulation. This produced a 31% decrease in DAMGO binding in layer I of area 24 but not in areas 24’ and 29 (LJ Vogt et al., 2001). Second, the midline and intralaminar thalamic nuclei and some nuclei that project to RSC express opioid receptors (LJ Vogt et al., 1992) and thalamic lesions reduce binding throughout the cingulate gyrus including a 24% reduction in layer Ia of area 29c (Vogt et al., 1995).
Expression of the µ-opioid receptor agonist Tyr-D-Ala-Gly-MePhe-Gly-ol (DAMGO) has been used for receptor binding and DAMGO-stimulated GTPgS stimulation with autoradiography to assess the overall composition of opioid circuits and provides insight into differentiation of pACC and MCC (L. Vogt et al., 2001). This study showed highest binding in area 32, intermediate amounts in areas 24 and 24´ and least in area 29 (Fig. 5), thus mimicking the regional differentiation observed in human brain. While area 24´ shared a similar laminar pattern of µ-opioid binding with area 24 (highest in layer V and moderate in layers I and VI), area 24´ shared a similar pattern of GTPgS stimulation with area 29 (moderate to low levels in layers I and V-VI). A correlation analysis of DAMGO binding and DAMGO-stimulated GTPgS activity confirmed that area 24´ has a unique opioid architecture from that of either areas 24 or 29. This confirms the regional differentiation of rodent cingulate cortex into pACC, MCC, and PCC.
Early studies reported a layer I concentration of µ-opioid receptors in ACC; however, experimental studies were needed to identify those components of the cortical neuropil that expressed the receptors in their dendrites and axonal terminals. Undercut lesions that remove all afferent axons, and therefore presynaptic receptors, show that about 30% layer II-VI binding is lost in area 24 and 50% is lost in layers I and V in area 29 (LJ Vogt et al., 2001; Vogt et al., 1995). The remaining binding following undercut lesions is expressed by the soma/dendritic membranes and possibly some glia. The question remains, however, which afferent axonal systems express µ-opioid receptors. There are at least two such sources. First, the locus coeruleus synthesizes µ-receptors and they are transported to presynaptic terminals in cingulate cortex. This was shown using the neurotoxin saporin conjugated to dopamine-b hydroxylase to kill neurons in the locus coeruleus followed by autoradiographic assay of binding and G-protein stimulation. This produced a 31% decrease in DAMGO binding in layer I of area 24 but not in areas 24’ and 29 (LJ Vogt et al., 2001). Second, the midline and intralaminar thalamic nuclei and some nuclei that project to RSC express opioid receptors (LJ Vogt et al., 1992) and thalamic lesions reduce binding throughout the cingulate gyrus including a 24% reduction in layer Ia of area 29c (Vogt et al., 1995).
Figure 5. Distribution of µ-opioid receptor binding with DAMGO autoradiography and activation of G-proteins with DAMGO and their assay with autoradiography for GTPgS. Highest binding and stimulation is in area 32 with progressively less in the rostrocaudal extent of cingulate cortex. This is similar to the topographic distribution of opioid receptor binding in primates. It is important that, although binding is higher in area 24b than area 24b´, the relative level of G-protein stimulation in area 24b´ is higher than in area 24b suggesting the opioid architecture and function in these two cingulate divisions is quite different. Although area 29c does not appear to have any direct role in pain processing, it does have µ-opioid receptors and G-protein stimulation and these regulate, among other systems, thalamocortical inputs because thalamic lesions greatly reduce ligand binding to these receptors (Vogt et al., 1995b).
Finally, nociceptive neurons in rat are in highest density in deep layers and most are pyramidal neurons with apical dendrites that distribute apical tufts in layer I (Yamamura et al., 1996). A nociceptive region is located in areas 32 and 24b in rat (Hsu et al., 2000) and rabbit (Sikes and Vogt, 1992) and this region is driven by electrical stimulation of the medial thalamus (Hsu et al., 1997). Thus, there are circuits in cingulate cortex for regulating motor behaviors as noted below and many of the cortical motor projection systems arise in layer V where there are highest levels of opioid receptor binding and most nociceptive neurons.
Finally, nociceptive neurons in rat are in highest density in deep layers and most are pyramidal neurons with apical dendrites that distribute apical tufts in layer I (Yamamura et al., 1996). A nociceptive region is located in areas 32 and 24b in rat (Hsu et al., 2000) and rabbit (Sikes and Vogt, 1992) and this region is driven by electrical stimulation of the medial thalamus (Hsu et al., 1997). Thus, there are circuits in cingulate cortex for regulating motor behaviors as noted below and many of the cortical motor projection systems arise in layer V where there are highest levels of opioid receptor binding and most nociceptive neurons.
Area 24b: Movement, Vision, and Pain Behaviors
Area 24b lays ventral to the medial agranular field (AGm) of Donoghue and Wise (1982) or Fr2/M2 in the rat (Zilles, 1985; Paxinos and Watson, 1986). Miller (1987) found corticospinal neurons mainly in areas 24b and 32 and these may be the rodent precursors of the cingulate motor areas in primates described by Morecraft and Van Hoesen (1992) and Dum and Strick (1993). AGm has strong connections with other motor areas, visual cortex, and retrosplenial area 29d (Reep et al., 1990) and there have been reports of low-threshold, contralateral head turning produced by electrical stimulation of area 24/24´ (Sinnamon and Galer, 1984). Unilateral lesions of AGm and area 24b impair approach to contralateral visual cues and transient sensory neglect (Vargo et al., 1988). In light of the major and reciprocal visual inputs to area 24b´ (Vogt and Miller, 1983; Miller and Vogt, 1984; Paperna and Malach, 1991) as well as posterior AGm (Reep et al., 1990), it is quite likely that area 24b is part of a visuomotor integration system.
Since lesions of AGm and area 24b´ alter hot plate reflexes in a condition termed "nocifensive apraxia" by Pastoriza et al. (1996), this rostral shoulder cortex may also be employed in generating avoidance behaviors in the context of nociceptive processing. Electrical stimulation of area 32 inhibits cardiovascular reactions in rats (Maskati and Zbrozyna (1989) and Vaccarino and Melzack (1989) were able to produce analgesia (i.e., reduced responses to tonic and phasic noxious stimulation) by injecting lidocaine into the cingulum bundle. Finally, Donahue et al. (2001) selectively blocked responses to inflammatory pain versus neuropathic pain with lesions in this region. Since area 24b has nociceptive neurons (Sikes and Vogt, 1992; Yamamura et al., 1996), it appears this area is involved in visual nocifensive processing as first suggested by Pastoriza et al. (1996). This also places the role of rat ACC (specifically area 24b) in pain processing in the domain of modulating motor functions. Since it has been suggested in monkey (Shima and Tanji, 1997) and human (Bush et al., 2002) that ACC is critical to changing the reward properties of behavior, including those associated with pain processing, area 24b appears to be pivotal to establishing the reward properties of a range of visually guided behaviors and may be critical to the prediction and avoidance of painful outcomes.
Since lesions of AGm and area 24b´ alter hot plate reflexes in a condition termed "nocifensive apraxia" by Pastoriza et al. (1996), this rostral shoulder cortex may also be employed in generating avoidance behaviors in the context of nociceptive processing. Electrical stimulation of area 32 inhibits cardiovascular reactions in rats (Maskati and Zbrozyna (1989) and Vaccarino and Melzack (1989) were able to produce analgesia (i.e., reduced responses to tonic and phasic noxious stimulation) by injecting lidocaine into the cingulum bundle. Finally, Donahue et al. (2001) selectively blocked responses to inflammatory pain versus neuropathic pain with lesions in this region. Since area 24b has nociceptive neurons (Sikes and Vogt, 1992; Yamamura et al., 1996), it appears this area is involved in visual nocifensive processing as first suggested by Pastoriza et al. (1996). This also places the role of rat ACC (specifically area 24b) in pain processing in the domain of modulating motor functions. Since it has been suggested in monkey (Shima and Tanji, 1997) and human (Bush et al., 2002) that ACC is critical to changing the reward properties of behavior, including those associated with pain processing, area 24b appears to be pivotal to establishing the reward properties of a range of visually guided behaviors and may be critical to the prediction and avoidance of painful outcomes.
Cortical Connections of Retrosplenial Cortex and Role in Visuospatial Function
The distribution of retrogradely labeled neurons following a horseradish peroxidase (HRP) injection into areas 29c/30 is presented in Figure 6. This case provided the first compelling demonstration of direct visual and retrosplenial cortex interactions and those with parahippocampal cortices (Miller and Vogt, 1983) and it now serves as the first to show differential inputs to these dorsal retrosplenial areas from pACC and MCC. There are three classes of cortical input to this region. First, direct visual sensory input arrives from the deep layers of areas 18a and b, but also area 17, as well as auditory cortex (Paperna and Malach, 1991). Second, the subiculum, deep layers of area 48, also termed the postsubiculum (Ps), and entorhinal cortex project to this region. Third, area 25 of pACC and MCC areas 24a´ and 24b´ project massively to areas 29c/30. It is surprising to see how little input to this region arises from areas 24a/b and how much arises from areas 24a'/b´.
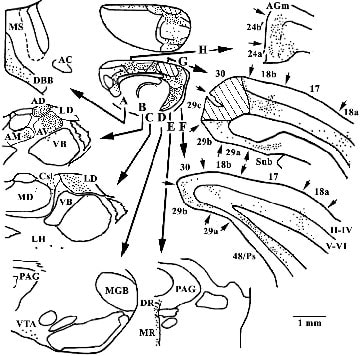
Figure 6. Retrogradely labeled neurons with HRP following an injection into areas 29c/30 (each dot represents about 3 labeled neurons). In addition to the three classes of cortical inputs (cingulate, parahip-pocampal, and sensory), notice that area 24´ has a greater projection than does area 24. There is a high level of input from the basal forebrain (DBB, diagonal band of Broca), anterior thalamic nuclei (AV, antero-ventral; AD, anterodorsal; AM, anteromedial) and laterodorsal (LD) and superior centrolateral (Csl) nuclei. The lateral hypo-thalamus (LH), ventral tegmental area (VTA), and raphe nuclei (DR, rorsal raphe; MR, median reaphe) also project prominently to this region. AC, anterior commisure; AGm, medial agranular motor cortex; VB, ventrobasal; MD, mediodorsal; MGB, medial geniculate nucleus; PAG, periaqueductal gray; Ps, postsubiculum; Sub, subiculum.
The HRP findings have been validated with injections of biotinylated dextran amine (BDA) into the same region and their cellular is origin shown in Figure 7. In particular there is the heavy and reciprocal connection between areas 24´ and RSC and much weaker input from area 24 (Fig. 7B, C). There also is massive intra-retrosplenial inputs from areas 29b and 29a (Fig 7D), and a major input from parahippocmpal cortex that includes the subiculum and entorhinal cortex (Fig. 7D, E).
The HRP findings have been validated with injections of biotinylated dextran amine (BDA) into the same region and their cellular is origin shown in Figure 7. In particular there is the heavy and reciprocal connection between areas 24´ and RSC and much weaker input from area 24 (Fig. 7B, C). There also is massive intra-retrosplenial inputs from areas 29b and 29a (Fig 7D), and a major input from parahippocmpal cortex that includes the subiculum and entorhinal cortex (Fig. 7D, E).
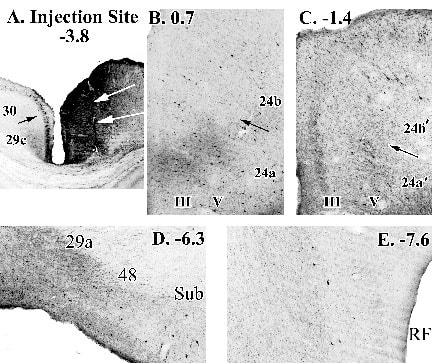
Figure 7. An injection of biotinylated dextran amine into areas 29c/30 (white arrows in A indicate cannula track) and retrogradely labeled neurons throughout cingulate and parahippocampal cortices. Of particular note is the higher level of input from area 24´ than area 24 and the dense innervation from area 29a and parahippocampal areas 48 and subiculum (D.) and less pronounced input from entorhinal cortex (E.).
Many observations support a significant role of area 29 in visuospatial functions. There is massive visual input to areas 29 and 30 and major projections from the postsubiculum which is involved in coding for head position in space (Taube et al., 1990). Indeed, RSC and visual area 18b both receive major inputs from the anteromedial nucleus of thalamus (Fig. 8; Rieck and Carey, 1985). This shared input likely assures that both areas have coordinated visuospatial processing. In addition, there are massive projections from the anterodorsal thalamic nucleus to area 29, the former of which is involved in coding spatial orientation according to background cues and radial-maze learning (Zugaro et al., 2001; Byatt and Dalrymple-Alford, 1996). Finally, many studies of visually guided behaviors support the visuospatial hypothesis of RSC function including those with the Morris water maze (Sutherland et al., 1988; Sutherland and Hoesing, 1993; Harker and Whishaw, 2002) and those showing that the late stages of acquisition of a visuospatial conditional discrimination is dependent on RSC (Bussey et al., 1997). Thus, parahippocampal, anterior thalamic, and visual cortical inputs to RSC provide the visual cues and orientation inputs necessary for neurons in this region to code for position of the body in space.
continue to Page 2
Many observations support a significant role of area 29 in visuospatial functions. There is massive visual input to areas 29 and 30 and major projections from the postsubiculum which is involved in coding for head position in space (Taube et al., 1990). Indeed, RSC and visual area 18b both receive major inputs from the anteromedial nucleus of thalamus (Fig. 8; Rieck and Carey, 1985). This shared input likely assures that both areas have coordinated visuospatial processing. In addition, there are massive projections from the anterodorsal thalamic nucleus to area 29, the former of which is involved in coding spatial orientation according to background cues and radial-maze learning (Zugaro et al., 2001; Byatt and Dalrymple-Alford, 1996). Finally, many studies of visually guided behaviors support the visuospatial hypothesis of RSC function including those with the Morris water maze (Sutherland et al., 1988; Sutherland and Hoesing, 1993; Harker and Whishaw, 2002) and those showing that the late stages of acquisition of a visuospatial conditional discrimination is dependent on RSC (Bussey et al., 1997). Thus, parahippocampal, anterior thalamic, and visual cortical inputs to RSC provide the visual cues and orientation inputs necessary for neurons in this region to code for position of the body in space.
continue to Page 2