Alzheimer’s Disease: A Cingulocentric View (1 of 2)
Much of this text is from an article written by
Brent A. Vogt, Alex Martin, Kent E. Vrana, Leslie J. Vogt & Patrick R. Hof
Multifocal Cortical Neurodegeneration in Alzheimer’s Disease:
In: Cerebral Cortex (Chapter 14; A. Peters and John H. Morrison, eds)
Kluwer Academic, New York
Brent A. Vogt, Alex Martin, Kent E. Vrana, Leslie J. Vogt & Patrick R. Hof
Multifocal Cortical Neurodegeneration in Alzheimer’s Disease:
In: Cerebral Cortex (Chapter 14; A. Peters and John H. Morrison, eds)
Kluwer Academic, New York
General Introduction
Many brain systems have been implicated in Alzheimer’s disease (AD). Damage to the hippocampus and parahippocampal cortex is often cited as the reason for early signs of memory and spatial impairments. Disruption of the cholinergic system has also been implicated in memory loss and a number of cholinergic drugs seek to remedy this symptom. Contrary to commonly held beliefs, however, early memory impairment in AD is associated with reduced glucose metabolism in posterior cingulate cortex (PCC; Satoshi Minoshima and his colleagues (1997; Annals of Neurology 42:85-94); before evidence of such hypometabolism can be detected in hippocampal and cholinergic systems. This is not to say that PCC is disrupted first in all cases of AD; it probably is not. Early glucose hypometabolism in PCC, however, indicates the importance of considering cingulate cortex in the early symptoms and disease progression and it raises the specter of a heterogeneous disease that has multiple variants and possibly subtypes.
Study of cingulate neurodegeneration in AD has led to the conclusion that this disease is very heterogeneous and may be comprised of as many as 5-10 clinicopathological variants. In the long run, it is in this region that multiple mechanisms of neurodegeneration will be isolated that will explain different patterns of neuron losses and associated patterns of clinical symptoms. These findings will eventually lead to methods for treating the many variants/subtypes in the Alzheimer and memory disorder clinics around the world.
Neurodegenerative processes in cingulate cortex are so important to Cingulum NeuroSciences Institute that the multivariate model of neuron death is part of our company logo. After reading the ensuing text, the meaning of the three dimensional graph and its 5 groups of colored dots (above) should be clear. Each dot is an individual AD case and its position in the graph is determined by the density of neurons in layers IIIab, IIIc, IV, and Va. If this were a single disease with a single progression, there would only be one large group of cases. Heterogeneity in neurodegeneration, however, produces statistically separable groups. When the mystery of neuron death in cingulate cortex is understood, we will have broken the code of this disease and be in a position to objectively and logically treat its many variants.
Study of cingulate neurodegeneration in AD has led to the conclusion that this disease is very heterogeneous and may be comprised of as many as 5-10 clinicopathological variants. In the long run, it is in this region that multiple mechanisms of neurodegeneration will be isolated that will explain different patterns of neuron losses and associated patterns of clinical symptoms. These findings will eventually lead to methods for treating the many variants/subtypes in the Alzheimer and memory disorder clinics around the world.
Neurodegenerative processes in cingulate cortex are so important to Cingulum NeuroSciences Institute that the multivariate model of neuron death is part of our company logo. After reading the ensuing text, the meaning of the three dimensional graph and its 5 groups of colored dots (above) should be clear. Each dot is an individual AD case and its position in the graph is determined by the density of neurons in layers IIIab, IIIc, IV, and Va. If this were a single disease with a single progression, there would only be one large group of cases. Heterogeneity in neurodegeneration, however, produces statistically separable groups. When the mystery of neuron death in cingulate cortex is understood, we will have broken the code of this disease and be in a position to objectively and logically treat its many variants.
Introduction to Cingulate Neurodegeneration
Cortical atrophy is well known in Alzheimer’s disease (AD), however, there are different interpretations of its location and extent. Neurodegeneration and atrophy occur in medical temporal areas, including hippocampal, entorhinal, and perirhinal cortices, and they follow a uniform pattern. This is not the only region of atrophy, however, since it occurs in prefrontal, parietotemporal, occipital, and cingulate cortices. Furthermore, although gross focal atrophy is not always present, focal neurodegeneration may occur with focal changes in glucose metabolism. The concept of multifocal cortical degeneration leads to the view of multiple structural and functional subsystem disruption in AD.
Although the argument will be made that multifocal cortical neurodegeneration accounts for clinical subgroups or variants, genetic and neuropathological observations suggest that differences among subgroups might reflect unique mechanisms of neurodegeneration and subtypes of the disease. To demonstrate subtypes, it must be shown that two or more symptoms are related to a corresponding number of unique etiologies (Jorm, 1985). Although the mechanisms of neurodegeneration in AD are not known, posterior cortical atrophy with Bálint syndrome-like presentation is an example of a likely subtype that has unique clinical presentations; multifocal atrophies in medical temporal, occipitoparietal, and posterior cingulate cortices; and impairments in visuomotor processing subsystems that differ from other AD cases. In another example, an exon 9 deletion in the presenilin 1 gene leads to a large senile plaques (SP) in the cerebral cortex, degeneration of corticospinal projection neurons, and spastic paraparesis (Crook et al., 1988). These and other examples of multifocal cortical atrophy with disruption of functional subsystems support the hypothesis that AD is comprised of subtypes.
A systematic methodology is not yet available whereby neuropathological analyses can assess subtypes at the molecular and cellular levels and relate such changes to alterations in brain metabolism and clinical outcomes. There may be genetic and neuropathological subtypes associated with different patterns of neurodegeneration within AD, yet this does not presume that links have been established to all clinical endpoints and are subtypes of AD. The subtype hypothesis is stated this way to provide a rationale for analyzing different pathological end points and provide a context for future clinical studies. Hence, the term subtypes as used here refers to a preliminary view that there are neuropathological subtypes within AD.
Selection of either the subgroup/variants or subtype hypothesis has a profound impact on study design and statistical analysis. Multiple biological subtypes suggest different patterns of transmitter system reorganization and that subgroups of patients will respond differently to drug therapies. Furthermore, this is a framework for hypotheses about brain-behavior relationships that are impaired in AD and lead to the goal of defining clinical subtypes based on specific patterns of neocortical neurodegeneration. Because neocortical neurodegeneration is not uniform in AD, and laminar patterns of neurodegeneration have a significant impact on transmitter systems, the pathological state of the cerebral cortex remains important whether or not neuropathological subtypes have yet been correlated with unique clinical endpoints. This review seeks to draw the line between subgroups and subtypes at the biological level. Subtypes cannot be defined solely on the basis of clinical and neuropsychological assessments, however, and genetic, glucose metabolism, and multifocal neurodegeneration are needed as an adjunct to support the subtypes hypothesis.
Although the argument will be made that multifocal cortical neurodegeneration accounts for clinical subgroups or variants, genetic and neuropathological observations suggest that differences among subgroups might reflect unique mechanisms of neurodegeneration and subtypes of the disease. To demonstrate subtypes, it must be shown that two or more symptoms are related to a corresponding number of unique etiologies (Jorm, 1985). Although the mechanisms of neurodegeneration in AD are not known, posterior cortical atrophy with Bálint syndrome-like presentation is an example of a likely subtype that has unique clinical presentations; multifocal atrophies in medical temporal, occipitoparietal, and posterior cingulate cortices; and impairments in visuomotor processing subsystems that differ from other AD cases. In another example, an exon 9 deletion in the presenilin 1 gene leads to a large senile plaques (SP) in the cerebral cortex, degeneration of corticospinal projection neurons, and spastic paraparesis (Crook et al., 1988). These and other examples of multifocal cortical atrophy with disruption of functional subsystems support the hypothesis that AD is comprised of subtypes.
A systematic methodology is not yet available whereby neuropathological analyses can assess subtypes at the molecular and cellular levels and relate such changes to alterations in brain metabolism and clinical outcomes. There may be genetic and neuropathological subtypes associated with different patterns of neurodegeneration within AD, yet this does not presume that links have been established to all clinical endpoints and are subtypes of AD. The subtype hypothesis is stated this way to provide a rationale for analyzing different pathological end points and provide a context for future clinical studies. Hence, the term subtypes as used here refers to a preliminary view that there are neuropathological subtypes within AD.
Selection of either the subgroup/variants or subtype hypothesis has a profound impact on study design and statistical analysis. Multiple biological subtypes suggest different patterns of transmitter system reorganization and that subgroups of patients will respond differently to drug therapies. Furthermore, this is a framework for hypotheses about brain-behavior relationships that are impaired in AD and lead to the goal of defining clinical subtypes based on specific patterns of neocortical neurodegeneration. Because neocortical neurodegeneration is not uniform in AD, and laminar patterns of neurodegeneration have a significant impact on transmitter systems, the pathological state of the cerebral cortex remains important whether or not neuropathological subtypes have yet been correlated with unique clinical endpoints. This review seeks to draw the line between subgroups and subtypes at the biological level. Subtypes cannot be defined solely on the basis of clinical and neuropsychological assessments, however, and genetic, glucose metabolism, and multifocal neurodegeneration are needed as an adjunct to support the subtypes hypothesis.
Neuropsychological Subgroups
The assessment of cognition has played a prominent role in investigations of AD because of the lack of a non-invasive biological marker that would support definitive diagnosis. One of the central findings that emerged from neuropsychological investigations was the discovery, or actually the rediscovery and clarification of the fact that clinically, AD is a heterogeneous disorder. Specifically, this claim refers to the fact that not all patients present with the same set of cognitive symptoms. Moreover, the differences between the types of patients under consideration here are not simply quantitative, and therefore not related to differences in the severity, disease duration, or rate of progression in any simple or straight forward manner. Rather, the claim of subgroups refers to the fact that AD patients can present with qualitatively distinct patterns of impaired and preserved cognitive abilities and skills. Thus, for example, one patient may have relatively severe visuospatial deficits concurrent with intact object naming and work-finding skills, whereas another patient may present with the opposite symptom profile. Groups of patients like these define qualitatively distinct subgroups, even though they all may have other symptoms in common, such as difficulty learning and remembering recently presented information. Moreover, and of critical importance for the present discussion, these different profiles are associated with correspondingly different profiles of cortical dysfunction as measured by positron emission tomography (PET). Thus, in the above example, the first patient would have more severe hypometabolism of the posterior parietal cortex (especially in the right hemisphere) relative to other cortical regions, whereas the second patient would have most-severe hypometabolism, limited to the left temporal lobe (see Martin, 1987, 1990, for reviews). These brain-imaging data provide a strong argument against the suggestion that individual patient profiles represent preexisting patterns of strengths and weaknesses that become exaggerated by a global pathological process.
How many subgroups or variants are there? If there were a biological marker that identified subjects prior to onset of overt, clinically relevant symptoms, the answer to this question would be obtained relatively easily. Once identified, serial evaluation of at-risk subjects would soon yield information on the following questions. First, what is the earliest symptom? Is an episodic or declarative memory problem always the first symptom to appear? If so, is this impairment global, affecting both verbal and non verbal processes-in which case it would indicate bilateral pathology of the medial temporal lobes or if the problem is material-specific, thus suggesting lateralized pathology? If other problems can precede impaired episodic memory, what are they? Can the initial symptom be a word-finding problem, suggesting focal involvement of the left temporal cortex, or a visuospatial problem that suggests focal parietal lobe disease? Second, regardless of the nature of the initial symptom, how does the dementia progress? Does progression always proceed in a fixed manner, or do different symptoms emerge in a seemingly random fashion?
The lack of a biological marker complicates our ability to answer these questions because the available data are tempered by two important constraints. The first constraint is defined by the type of symptoms that motivate people to present themselves for evaluation. Some cognitive deficits, such as language deficits and verbal memory problems, have considerably greater impact on activities of daily living than other symptoms, such as nonverbal object-recognition and memory problems. As a result, focal involvement of the lateral and medial aspects of the left temporal lobe may be seen early in the course of the disease more often than focal involvement of the right temporal lobe. Yet it would be a mistake to conclude that this asymmetry indicates greater vulnerability to AD of the left than right temporal lobe. Thus, the true incidence of the types of cognitive problems present during the earliest stages of the disease may be distorted, and the likelihood of identifying the full range of preclinical deficits is reduced by this symptom presentation bias.
The second constraint is dictated not by the patients but by the clinician. Current diagnostic criteria for probable AD demand that the patient be impaired in at least two domains of cognition. There is no question that this is a valid diagnostic criterion in that it increases the probability of a correct diagnosis and greatly reduces false positives. Nevertheless, it should also be apparent that this criterion eliminates patients who initially present with a single deficit. Thus, the absence of a diagnostic marker has let to both patient-based and clinician-based biases that, in turn, may constrain and potentially distort answers to questions about the range of cognitive deficits associated with the initial presentation of AD, its progression, and the number of qualitatively distinguishable subgroups.
With these caveats in mind, it can be confidently stated that AD typically is associated with dysfunction in primarily four cognitive domains: (1) Deficits in episodic memory, which are assessed by tests of learning and remembering verbal and nonverbal material and are associated with bilateral involvement of the medial temporal lobe, particularly the hippocampus and parahippocampal, perirhinal and entorhinal cortices; (2) Deficits in semantic memory, which are assessed by tests of object naming and word knowledge and are associated with involvement of the left temporal cortex, and also deficits in nonverbal object recognition as assessed by tests of recognition and pattern matching, which are associated with involvement of the right temporal cortex; (3) Deficits in visuospatial abilities and skills as assessed by tests of spatial attention, object localization, orientation, and constructional ability and are associated with involvement of the parietal lobes; (4) Deficits in regulating and controlling thought and behavior and in manipulating the products of memory, as evidenced by personality changes and social/occupational dysfunction and assessed by measures of problem solving, conceptualization, and working memory. This last constellation of symptoms, which are often associated with frontal lobe involvement, can emerge early in the course of the disease, as suggested by Royall et al. (1994) and by the case presented below that displays frontal lobe involvement and early disruption of working memory and problem solving associated with paranoid behaviors. Consistent with this framework, PET has shown that cerebral hypometabolism occurs in the medial temporal region, the posterior region of the temporal and parietal lobes, the PCC and, to a lesser extent, the prefrontal cortex (Haxby et al., 1988). Because glucose hypometabolism in patients with very early AD is greatest in PCC (Minoshima et al., 1997), PCC may play a greater role in memory and visuospatial deficits than generally appreciated.
Within this general framework, single cases present with contrasting patterns of impaired and preserved cognitive difficulties that may focus on any single cognitive domain, especially during the early stages of the disease. Typically, and perhaps as a result of diagnostic criteria, patients have difficulty learning and remembering recently presented material. In addition to memory impairment, the best-documented subgroups consist of patients with deficits in semantic memory and word-finding ability, concurrent with normal or near-normal visuospatial skills, and patients with the opposite profile of impaired and preserved abilities (Becker et al., 1988; Fisher et al., 1996; Haxby et al., 1985; Martin et al., 1986). Disease progression in these relatively focal cases is characterized by tow factors: continued deterioration with the affected cognitive domain and, by implication, within its functional brain substrate (Martin et al., 1987) and spreading or widening of symptoms to other, previously intact domains (Becker et al., 1988). The fact that the disease continues to progress within a single cognitive domain suggests that the progression of pathology is not random, but rather carves a course that appears to parallel the functional organization of the affected system (Martin et al., 1987).
As noted above, these patients are also characterized by contrasting patterns of cerebral hypometabloism in the expected regions, based on their most prominent cognitive deficit (Foster et al., 1983; Haxby et al., 1985; Martin et al., 1986). Specifically, left temporal lobe hypometabolism in patients with object-naming deficits and other semantic memory problems, and parietal lobe hypometabolism, often more pronounced on the right than the left, in patients with visuospatial deficits. As we noted previously (Martin et al., 1987, 1990), these brain-imaging data are consistent with the functional neuroanatomy of object and spatial vision, with object perception and knowledge mediated largely by the ventral, occipitotemporal processing stream, and with spatial perception and spatial cognition mediated by the dorsal, occipitoparietal processing stream (Ungerlieder and Mishkin, 1982; see Ungerlieder and Haxby, 1994, for review). Thus, these visual processing systems seem to be at greater risk than other functional systems for developing AD neuropathology, and can be affected in relative isolation from each other in individual cases.
In addition, it appears that even within one of these subgroups, patients can differ with regard to important characteristics. For example, as noted above, many of the patients with prominent visuospatial difficulties have little difficulty in visually recognizing objects. They perform at near normal levels on tests of object naming and face recognition, while being profoundly impaired on tests that require spatial analyses, such as route finding, judging the orientation of a line, or copying abstract designs (Becker et al., 1988; Fisher et al., 1966; Martin et al., 1986). It is this pattern of deficits that is associated with severe posterior parietal hypometabolism relative to other neocortical regions. The fact that these patients can name line drawings of objects indicates that early visual processing stages are intact, and indeed, in these patients, PET reveals relative sparing of the occipital lobes. In contrast, other patients show a more profound visual disorder that affects basic visual processes and thus results in both spatial impairments and object recognition deficits (Levine et al., 1993; Furey-Kurkjian et al., 1996) (Fig. 1). Studies of these visually impaired cases have raised two important question about variability of pathology associated with AD. First, is the hippocampus and adjacent cortex invariable involved during the early states of the disease? Second, are primary sensory and motor areas always better preserved than parasensory and limbic association cortices?
At autopsy the medial temporal lobes contain SP and NFT as documented in many studies (Hirano and Zimmerman, 1962; Van Hoesen et al., 1991; Braak and Braak, 1991; Hof et al., 1992; Gómez-Isla et al., 1996). Significant memory problems commonly occur in typical cases, as well as in the above-described cases, with relatively focal language and visuospatial impairments. There have also been a number of reports of autopsy-documented cases of AD that initially presented with an isolated amnesic syndrome that lasted several years before progressing into a global dementia (Neary et al., 1986). These findings suggest that the medial temporal region may be the only region involved early in the disease, but leaves open the question of whether the disease can initially begin outside of this region. Reports from the visually impaired patients and their families indicate that the visual problems were the earliest and sole symptom experienced by these patients, interfering with daily activities long before the onset of clinically apparent memory difficulties (Crystal et al., 1992; Levine et al., 1993; Pietrini et al., 1996). This suggests that the hippocampus and adjacent structures were not the initial site of AD-associated pathology in these cases. Consistent with these clinical reports, the psychometric data have shown that the visual impairments were markedly more severe than the memory problems in these patients. Nevertheless, it must be noted that when formally tested, memory functions have not been normal, even for verbal material presented auditorily (Furey-Kurkjian et al., 1996). Since PCC is impaired very early (Minoshima et al., 1997), it remains to be documented that episodic memory functions and associated medial temporal lobe structures can be truly unaffected in the early stages of the disease in some cases or whether disruption at other sites accounts for impaired memory.
How many subgroups or variants are there? If there were a biological marker that identified subjects prior to onset of overt, clinically relevant symptoms, the answer to this question would be obtained relatively easily. Once identified, serial evaluation of at-risk subjects would soon yield information on the following questions. First, what is the earliest symptom? Is an episodic or declarative memory problem always the first symptom to appear? If so, is this impairment global, affecting both verbal and non verbal processes-in which case it would indicate bilateral pathology of the medial temporal lobes or if the problem is material-specific, thus suggesting lateralized pathology? If other problems can precede impaired episodic memory, what are they? Can the initial symptom be a word-finding problem, suggesting focal involvement of the left temporal cortex, or a visuospatial problem that suggests focal parietal lobe disease? Second, regardless of the nature of the initial symptom, how does the dementia progress? Does progression always proceed in a fixed manner, or do different symptoms emerge in a seemingly random fashion?
The lack of a biological marker complicates our ability to answer these questions because the available data are tempered by two important constraints. The first constraint is defined by the type of symptoms that motivate people to present themselves for evaluation. Some cognitive deficits, such as language deficits and verbal memory problems, have considerably greater impact on activities of daily living than other symptoms, such as nonverbal object-recognition and memory problems. As a result, focal involvement of the lateral and medial aspects of the left temporal lobe may be seen early in the course of the disease more often than focal involvement of the right temporal lobe. Yet it would be a mistake to conclude that this asymmetry indicates greater vulnerability to AD of the left than right temporal lobe. Thus, the true incidence of the types of cognitive problems present during the earliest stages of the disease may be distorted, and the likelihood of identifying the full range of preclinical deficits is reduced by this symptom presentation bias.
The second constraint is dictated not by the patients but by the clinician. Current diagnostic criteria for probable AD demand that the patient be impaired in at least two domains of cognition. There is no question that this is a valid diagnostic criterion in that it increases the probability of a correct diagnosis and greatly reduces false positives. Nevertheless, it should also be apparent that this criterion eliminates patients who initially present with a single deficit. Thus, the absence of a diagnostic marker has let to both patient-based and clinician-based biases that, in turn, may constrain and potentially distort answers to questions about the range of cognitive deficits associated with the initial presentation of AD, its progression, and the number of qualitatively distinguishable subgroups.
With these caveats in mind, it can be confidently stated that AD typically is associated with dysfunction in primarily four cognitive domains: (1) Deficits in episodic memory, which are assessed by tests of learning and remembering verbal and nonverbal material and are associated with bilateral involvement of the medial temporal lobe, particularly the hippocampus and parahippocampal, perirhinal and entorhinal cortices; (2) Deficits in semantic memory, which are assessed by tests of object naming and word knowledge and are associated with involvement of the left temporal cortex, and also deficits in nonverbal object recognition as assessed by tests of recognition and pattern matching, which are associated with involvement of the right temporal cortex; (3) Deficits in visuospatial abilities and skills as assessed by tests of spatial attention, object localization, orientation, and constructional ability and are associated with involvement of the parietal lobes; (4) Deficits in regulating and controlling thought and behavior and in manipulating the products of memory, as evidenced by personality changes and social/occupational dysfunction and assessed by measures of problem solving, conceptualization, and working memory. This last constellation of symptoms, which are often associated with frontal lobe involvement, can emerge early in the course of the disease, as suggested by Royall et al. (1994) and by the case presented below that displays frontal lobe involvement and early disruption of working memory and problem solving associated with paranoid behaviors. Consistent with this framework, PET has shown that cerebral hypometabolism occurs in the medial temporal region, the posterior region of the temporal and parietal lobes, the PCC and, to a lesser extent, the prefrontal cortex (Haxby et al., 1988). Because glucose hypometabolism in patients with very early AD is greatest in PCC (Minoshima et al., 1997), PCC may play a greater role in memory and visuospatial deficits than generally appreciated.
Within this general framework, single cases present with contrasting patterns of impaired and preserved cognitive difficulties that may focus on any single cognitive domain, especially during the early stages of the disease. Typically, and perhaps as a result of diagnostic criteria, patients have difficulty learning and remembering recently presented material. In addition to memory impairment, the best-documented subgroups consist of patients with deficits in semantic memory and word-finding ability, concurrent with normal or near-normal visuospatial skills, and patients with the opposite profile of impaired and preserved abilities (Becker et al., 1988; Fisher et al., 1996; Haxby et al., 1985; Martin et al., 1986). Disease progression in these relatively focal cases is characterized by tow factors: continued deterioration with the affected cognitive domain and, by implication, within its functional brain substrate (Martin et al., 1987) and spreading or widening of symptoms to other, previously intact domains (Becker et al., 1988). The fact that the disease continues to progress within a single cognitive domain suggests that the progression of pathology is not random, but rather carves a course that appears to parallel the functional organization of the affected system (Martin et al., 1987).
As noted above, these patients are also characterized by contrasting patterns of cerebral hypometabloism in the expected regions, based on their most prominent cognitive deficit (Foster et al., 1983; Haxby et al., 1985; Martin et al., 1986). Specifically, left temporal lobe hypometabolism in patients with object-naming deficits and other semantic memory problems, and parietal lobe hypometabolism, often more pronounced on the right than the left, in patients with visuospatial deficits. As we noted previously (Martin et al., 1987, 1990), these brain-imaging data are consistent with the functional neuroanatomy of object and spatial vision, with object perception and knowledge mediated largely by the ventral, occipitotemporal processing stream, and with spatial perception and spatial cognition mediated by the dorsal, occipitoparietal processing stream (Ungerlieder and Mishkin, 1982; see Ungerlieder and Haxby, 1994, for review). Thus, these visual processing systems seem to be at greater risk than other functional systems for developing AD neuropathology, and can be affected in relative isolation from each other in individual cases.
In addition, it appears that even within one of these subgroups, patients can differ with regard to important characteristics. For example, as noted above, many of the patients with prominent visuospatial difficulties have little difficulty in visually recognizing objects. They perform at near normal levels on tests of object naming and face recognition, while being profoundly impaired on tests that require spatial analyses, such as route finding, judging the orientation of a line, or copying abstract designs (Becker et al., 1988; Fisher et al., 1966; Martin et al., 1986). It is this pattern of deficits that is associated with severe posterior parietal hypometabolism relative to other neocortical regions. The fact that these patients can name line drawings of objects indicates that early visual processing stages are intact, and indeed, in these patients, PET reveals relative sparing of the occipital lobes. In contrast, other patients show a more profound visual disorder that affects basic visual processes and thus results in both spatial impairments and object recognition deficits (Levine et al., 1993; Furey-Kurkjian et al., 1996) (Fig. 1). Studies of these visually impaired cases have raised two important question about variability of pathology associated with AD. First, is the hippocampus and adjacent cortex invariable involved during the early states of the disease? Second, are primary sensory and motor areas always better preserved than parasensory and limbic association cortices?
At autopsy the medial temporal lobes contain SP and NFT as documented in many studies (Hirano and Zimmerman, 1962; Van Hoesen et al., 1991; Braak and Braak, 1991; Hof et al., 1992; Gómez-Isla et al., 1996). Significant memory problems commonly occur in typical cases, as well as in the above-described cases, with relatively focal language and visuospatial impairments. There have also been a number of reports of autopsy-documented cases of AD that initially presented with an isolated amnesic syndrome that lasted several years before progressing into a global dementia (Neary et al., 1986). These findings suggest that the medial temporal region may be the only region involved early in the disease, but leaves open the question of whether the disease can initially begin outside of this region. Reports from the visually impaired patients and their families indicate that the visual problems were the earliest and sole symptom experienced by these patients, interfering with daily activities long before the onset of clinically apparent memory difficulties (Crystal et al., 1992; Levine et al., 1993; Pietrini et al., 1996). This suggests that the hippocampus and adjacent structures were not the initial site of AD-associated pathology in these cases. Consistent with these clinical reports, the psychometric data have shown that the visual impairments were markedly more severe than the memory problems in these patients. Nevertheless, it must be noted that when formally tested, memory functions have not been normal, even for verbal material presented auditorily (Furey-Kurkjian et al., 1996). Since PCC is impaired very early (Minoshima et al., 1997), it remains to be documented that episodic memory functions and associated medial temporal lobe structures can be truly unaffected in the early stages of the disease in some cases or whether disruption at other sites accounts for impaired memory.
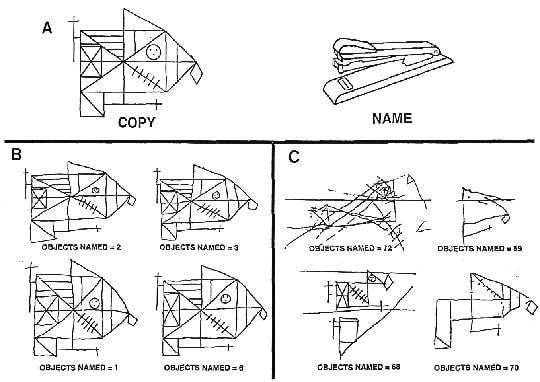
Figure 1. Examples of visuospatial/constructional skill and object-naming ability in AD patients forming qualitatively distinct subgroups. (A) On the left is a complex, meaningless figure commonly used to test visuospatial and constructional skill (Osterrieth, 1944). On the right is an example of 1 of 85 objects used to test object-naming ability (Kaplan et al., 1976). (B) Four AD patients with intact visuoconstructional skills and severely impaired object-naming ability. (C) Four patients with the opposite cognitive profile. Relative to other cortical regions, PET revealed substantial left temporal lobe glucose hypometabolism for the patients in panel B and substantial right parietal glucose hypometabolism for the patients in panel C. AD was verified at autopsy in at least one patient in each subgroup.
The visually impaired patients also show that, contrary to common belief, AD can affect a primary sensory area. In fact, the data suggest that primary visual cortex cam be the most impaired region and perhaps the site of initial pathology. Autopsy studies have verified that the visually impaired patients have SP and NFT in the occipital lobe, including calcarine cortex, as discussed in detail below. PET studies have revealed that the most severe hypometabolism is in the dorsal visual stream, extending from the calcarine region to posterior parietal cortex (Pietrini et al., 1996). Such cases raise another question about the regional variation of AD that has potential etiological significance: why, in at least some cases, does AD affect primary visual, but not primary auditory, or somatosensory cortices?
The PCC has been implicated in every postmortem study that evaluated this region in terms of neurodegeneration, alterations in the cholinergic, serotonergic and noradrenergic systems, and PET studies. Futhermore, cases of posterior cortical atrophy in AD have a more severe neurodegeneration and tau immunoreactivity in PCC than they do in posterior parietal cortex (Hof et al., 1997; Vogt et al., 1997). In spite of these early and profound changes, the involvement of PCC in AD is poorly appreciated, and this region is not considered in routine neuropathological assessments of the disease. One reason for this oversight is the lack of neuropsychological tests to elucidate impairments of cingulate cortex function, including those that test the specific contribution of this region to visuospatial function, reviewed by Olson et al., (1993). It is also possible that early neurodegeneration in PCC does not always involve deposition of classical lesions but undergoes neuropathological assessments.
The conundrum of current AD investigations is that having observed subgroups with joint clinical and cerebrally localized impairments of glucose metabolism, postmortem assessments simply verify the presence of a single disease based on established criteria. There are no systematic methodologies for assessing the extent to which subgroups represent unique subtypes. The extent to which subgroups based on neuropsychological testing represent unique subtypes cannot be determined from testing and PET studies alone, and it is not known how many different mechanisms of neurodegeneration there are in AD. Thus, although defining clinically unique subtypes of AD largely remains a goal, the evidence for multifocal degeneration is clear and biological subtypes can be assessed and should dovetail with clinical analyses based on more refined neuropsychological testing.
The visually impaired patients also show that, contrary to common belief, AD can affect a primary sensory area. In fact, the data suggest that primary visual cortex cam be the most impaired region and perhaps the site of initial pathology. Autopsy studies have verified that the visually impaired patients have SP and NFT in the occipital lobe, including calcarine cortex, as discussed in detail below. PET studies have revealed that the most severe hypometabolism is in the dorsal visual stream, extending from the calcarine region to posterior parietal cortex (Pietrini et al., 1996). Such cases raise another question about the regional variation of AD that has potential etiological significance: why, in at least some cases, does AD affect primary visual, but not primary auditory, or somatosensory cortices?
The PCC has been implicated in every postmortem study that evaluated this region in terms of neurodegeneration, alterations in the cholinergic, serotonergic and noradrenergic systems, and PET studies. Futhermore, cases of posterior cortical atrophy in AD have a more severe neurodegeneration and tau immunoreactivity in PCC than they do in posterior parietal cortex (Hof et al., 1997; Vogt et al., 1997). In spite of these early and profound changes, the involvement of PCC in AD is poorly appreciated, and this region is not considered in routine neuropathological assessments of the disease. One reason for this oversight is the lack of neuropsychological tests to elucidate impairments of cingulate cortex function, including those that test the specific contribution of this region to visuospatial function, reviewed by Olson et al., (1993). It is also possible that early neurodegeneration in PCC does not always involve deposition of classical lesions but undergoes neuropathological assessments.
The conundrum of current AD investigations is that having observed subgroups with joint clinical and cerebrally localized impairments of glucose metabolism, postmortem assessments simply verify the presence of a single disease based on established criteria. There are no systematic methodologies for assessing the extent to which subgroups represent unique subtypes. The extent to which subgroups based on neuropsychological testing represent unique subtypes cannot be determined from testing and PET studies alone, and it is not known how many different mechanisms of neurodegeneration there are in AD. Thus, although defining clinically unique subtypes of AD largely remains a goal, the evidence for multifocal degeneration is clear and biological subtypes can be assessed and should dovetail with clinical analyses based on more refined neuropsychological testing.
Genetic Factors: Subgroups or Subtypes?
Five genetic loci cosegregate with familial and sporadic forms of AD (Hardy, 1966; Yankner, 1995, for reviews) and inherited and somatic mutations of mitochondrial DNA occur in AD (Shoffner et al., 1993). These genes encode fundamentally different proteins that subserve disparate functions, and although it is possible that these genes converge on a single end-stage pathology, it is more likely that they do so via distinct pathways early in the disease and in distinct regional and laminar patterns. Our premise, therefore, is that different genetic variables contribute to distinct neuropathologies such as those associated with presenilin-1 (PS) and amyloid precursor protein (APP) genes. The strongest evidence for the concept of genetically linked subtypes is spastic paraparesis, degeneration of the corticospinal tract, and large SP without cores following deletion of exon 9 from the PS1 gene (Crook et al., 1998). It may also be true that mitochondrial DNA mutations lead to selective vulnerability in regions with high levels of oxidative metabolism such as the retrosplenial areas of PCC (Matsunami et al., 1989). Indeed, this region experiences the first glucose hypometabolism early in AD (Minoshina et al., 1997). Furthermore, although homozygous inheritance of the e2 and e4 alleles with frank genetic missense mutation in other genes suggests synergistic genetic subtypes and a potential for neuropathological and clinical subtypes associated with each.
Frank Genetic Subtypes: Autosomal Dominant Mutations
There are four classes of heritable genetic mutations associated with AD: mutations in the APP, in one of the two PS genes, mtDNA variants, and the recent identification of anew inherited genetic risk for late-onset AD on chromosome 12 (Pericak-Vance et al., 1997). The APP is a large membrane-spanning protein of unknown function. It serves as the precursor for the 40-43-amino acid amyloid b-peptide (Ab), which itself is a major component of SP. The APP gene is on chromosome 21 and has been implicated in AD through genetic analyses of rare familial forms of early-onset AD and Down’s syndrome. There are five documented autosomal dominant forms of inherited, early-onset AD that are associated with mutations in the APP gene (see Hardy, 1997, for review). Although the number of families affected by APP mutations is small, the nature of the mutations provides insight into the disease process and has let to animal models of AD (Games et al., 1995; LaFerla et al., 1995). All of the mutations cluster near the site encoding the Ab processed peptide and it is thought that miscleavage of APP results in either an increased production of Ab and/or formation of a less soluble form of the processed peptide.
Amyloid b-peptides are potent neurotoxins in primary CNS culture and clonal cell lines. Ab42 and Ab25-35 induce apoptosis in hippocampal neurons (Forloni et al., 1993; Loo et al., 1993). Although Ab40 can induce apoptosis in cultured rat cortical neurons (Estus et al., 1997), it is the aggregation of this peptide that is particularly important. The toxic intermediates of Ab may be free radicals. Ab25-35 induces lipid peroxidation (Kruman et al., 1997) and increases hydrogen peroxide and lipid peroxides (Behl et al., 1994), which themselves induce oxidative damage of mitochondrial DNA (Bozner et al., 1997). Finally, as discussed below for cases of focal cortical atrophy, Ab42 is the highest in layers II-IIIab of prefrontal cortex with most severe neuron losses. The progressive involvement of visual cortical areas in posterior cortical atrophy also suggests that increasing neurodegeneration is associated with a graded increase in the levels of Ab (Hof et al., 1997).
Missense mutations in the PS genes co-segregate with early-onset AD (Clark et al., 1995; Levy-Lahad et al., 1995; Hutton et al., 1996). The PSs are transmembrane proteins thought to contain seven or eight membrane-spanning domains (Li and Greenwald, 1996; Doan et al., 1996). The two related genes in this family reside on chromosome 14 (PS1) and chromosome 1 (PS2). While the functions of these proteins remain obscure, their mutation is associated with autosomal dominant forms of early-onset AD. There are now more than 40 genetic mutations that, with the exception of a single case of a deletion leading to altered PS1 mRNA splicing, convert a single amino acid to another amino acid. Mutations in the PSs lead to increased insoluble Ab production (Scheuner et al., 1996; Brochelt et al., 1996; Citron et al., 1997). Moreover, Guo et al. (1997) demonstrated that PS1 mutations increase Ab-induced apoptosis, and Maury et al. (1997) reported that PS mutations themselves can produce amyloidogenic peptides.
Amyloid b-peptides are potent neurotoxins in primary CNS culture and clonal cell lines. Ab42 and Ab25-35 induce apoptosis in hippocampal neurons (Forloni et al., 1993; Loo et al., 1993). Although Ab40 can induce apoptosis in cultured rat cortical neurons (Estus et al., 1997), it is the aggregation of this peptide that is particularly important. The toxic intermediates of Ab may be free radicals. Ab25-35 induces lipid peroxidation (Kruman et al., 1997) and increases hydrogen peroxide and lipid peroxides (Behl et al., 1994), which themselves induce oxidative damage of mitochondrial DNA (Bozner et al., 1997). Finally, as discussed below for cases of focal cortical atrophy, Ab42 is the highest in layers II-IIIab of prefrontal cortex with most severe neuron losses. The progressive involvement of visual cortical areas in posterior cortical atrophy also suggests that increasing neurodegeneration is associated with a graded increase in the levels of Ab (Hof et al., 1997).
Missense mutations in the PS genes co-segregate with early-onset AD (Clark et al., 1995; Levy-Lahad et al., 1995; Hutton et al., 1996). The PSs are transmembrane proteins thought to contain seven or eight membrane-spanning domains (Li and Greenwald, 1996; Doan et al., 1996). The two related genes in this family reside on chromosome 14 (PS1) and chromosome 1 (PS2). While the functions of these proteins remain obscure, their mutation is associated with autosomal dominant forms of early-onset AD. There are now more than 40 genetic mutations that, with the exception of a single case of a deletion leading to altered PS1 mRNA splicing, convert a single amino acid to another amino acid. Mutations in the PSs lead to increased insoluble Ab production (Scheuner et al., 1996; Brochelt et al., 1996; Citron et al., 1997). Moreover, Guo et al. (1997) demonstrated that PS1 mutations increase Ab-induced apoptosis, and Maury et al. (1997) reported that PS mutations themselves can produce amyloidogenic peptides.
Clinical and Neuropathological Outcomes Following PS and APP Mutations
Comparison of clinical outcomes associated with autosomal dominant mutations in the APP and PS genes suggests that they are different and may have differences in underlying pathology. The APP mutations (Lantos et al., 1992; Mann et al., 1992; Cairns et al., 1993; Mullan et al., 1993) are associated with earlier onset than are PS mutations (<50 vs. >50), more rapid progression, early progressive aphasia, and more frequent myoclonus, seizures, and psychosis (Haltia et al., 1994; Lampe et al., 1994; Kennedy et al., 1995; Fox et al., 1997; Harvey et al., 1998). The brains of individuals with APP mutations are said to be "entirely typical," although they have elevated amyloid burden (above citations). In contrast, two cases with PS mutations were "unusual microscopically and both showed symmetric cortical atrophy which was particularly severe in the frontal and temporal lobes" (Harvey et al., 1998, p. 46). Other cases have been reported with moderate spongiform changes (Haltia et al., 1994) and high levels of Ab42 (Haltia et al., 1994; Lampe et al., 1994; Gómez-Isla et al., 1997).
The most profound finding of a relationship between an autosomal dominant mutation and unique patterns of clinical and neuropathological outcomes has been the consequences of deletion of exon 9 in the PS1 gene (Crook et al., 1998). This mutation results in early spastic paraparesis associated with large SP without cores throughout the cerebral cortex and degeneration of the corticospinal tract. Although motor cortex is not generally impacted in AD, these cases show that even this region is at risk in some patients. Although some of the studies discussed above do not employ a large number of cases, all of the findings together suggest that correlative autosomal dominant mutations are associated with different patterns of Ab deposition, neurodegeneration, and clinical outcomes. As these findings are solidified, this may provide important confirmation of the subtypes hypothesis of AD.
The most profound finding of a relationship between an autosomal dominant mutation and unique patterns of clinical and neuropathological outcomes has been the consequences of deletion of exon 9 in the PS1 gene (Crook et al., 1998). This mutation results in early spastic paraparesis associated with large SP without cores throughout the cerebral cortex and degeneration of the corticospinal tract. Although motor cortex is not generally impacted in AD, these cases show that even this region is at risk in some patients. Although some of the studies discussed above do not employ a large number of cases, all of the findings together suggest that correlative autosomal dominant mutations are associated with different patterns of Ab deposition, neurodegeneration, and clinical outcomes. As these findings are solidified, this may provide important confirmation of the subtypes hypothesis of AD.
Mitochondrial DNA Mutations
Variants in mitochondrial DNA are maternally transmitted and also accumulate with age, particularly in the eighth decade (Corral-Debrinski et al., 1992). Among other things, these genes encode transfer RNA in the mitochondria. The somatic mutations could result form oxygen free-radical damage. Although the functional consequences of such mutations are unknown, it is possible that disruption of mitochondrial function and passage of similar mutations to successive generations of mitochondria within the neuron may lead to significantly impaired oxidative phosphorylation and possibly cell death as a consequence of overproduction of oxygen free radicals. Because reductions in PCC neurons between the ages of 55 and 95 do not occur in neurologically intact individuals (Vogt et al., 1998), it is likely that a combination of age-related and inherited mutations (Schoffner et al., 1993) are responsible for neurodegenerative processes in AD.
Genetic Risk Factor: The ApoE Gene
Unlike the APP and PS genes, the ApoE gene on chromosome 19 does not act as the locus for the autosomal dominant mutations that lead to AD. Rather, the three naturally occurring alleles for this gene modulate the likelihood of being afflicted with the disease. The e4 allele that is rare in the general population is a risk factor for AD, while the e2 allele may be protective and is found much less frequently in AD than in the general population (Corder et al., 1994). Biochemical explanations for the functional role of the ApoE gene in the etiology of AD include a differential interaction with the tau protein in NFT and with Ab.
There are a number of different ways to consider the risk liability of ApoE genotype. It has been suggested that the homozygous e4 individual has an eightfold greater risk of AD (Roses, 1995). Another statistical treatment suggests that this same genotype increases the risk by 10 years, with a single allele contributing one-half of this value. Another estimate is that the ApoE genetic locus accounts for approximately 50% of the attributable risk (Nalbantoglu et al., 1994). In contrast, a study of 310 families by Blacker et al. (1997) reported the greatest risk in early on-set cases and no role for e4 homozygotes between ages 60 and 65, suggesting greatest risk in early-onset cases and no role for transmission of a single e4 allele. Attempts to directly link ApoE genotype and AD neuropathology have shown only an increase in SP in e4 homozygotes, and the e4 risk liability is not limited to AD but includes critical coronary heart disease, hypertension, and epilepsy, as discussed below. The crucial question in relation to AD is, what other genes are responsible for focal neocortical pathology?
The essential argument for genetic subtypes and preliminary indications of their neuropathological impact arise from the interaction of different ApoE alleles on other genetic systems. While homozygous expression of the e4 allele increases the risk for AD, it exerts differential modulatory effects on other genes. The e4 allele decreases the age at onset for early-onset AD associated with APP mutations (Houlden et al., 1993), whereas the e4 allele may not have the same effect on the early-onset disease associated with presenilin mutations (Van Broeckhoven et al., 1994). The same gene, therefore, exerts different effects depending on the genetic environment. Conversely, the e2 allele may be protective against AD and so this allele is underrepresented in AD populations (Talbot et al., 1994; Corder et al., 1994). There are a number of plausible molecular explanations for this finding based on binding of the tau protein in NFT and interactions with Ab. However, this protective effect is absent in early-onset AB (Maestre et al., 1995) and in black populations (van Duijn et al., 1994). Thus, expression of the ApoE alleles influences the onset and progression of AD via their interactions with the products of other genes. For example, the e4 allele is associated with higher densities of SP in AD (Schmechel et al., 1993; Rebeck et al., 1993), and these SP have greater amounts of Ab40 which is not fibrillogenic (Gearing et al., 1996). However, PS1 mutations elevate the longer form of Ab (Borchelt et al., 1996; Scheuner et al., 1996), suggesting that homozygous expression of the ApoE e4 allele and a PS1 mutation will increase SP densities still further.
Studies of PCC suggest that, although homozygous expression of the ApoE e4 allele is associated with greater numbers of SP, the e4 risk is associated only with the most severe neurodegeneration and does not account for most laminar patterns in degeneration (Vogt et al., 1998). Because the ApoE risk is associated with altered processing of the APP throughout the body, including cortical deposition of SP{ in non-demented individuals with critical coronary heart disease and hypertension (Sparks et al., 1996), and in epileptogenic cortex (Gouras et al., 1997), the significance of the ApoE risk may be its influence on lipid metabolism. The issue raised by genomic studies of the ApoE is to what extent other genes may be responsible for neocortical pathology including disease subtypes and how the functions of these unknown genes are altered in e4 homozygotes. For example, Jordan et al. (1998) provide evidence that ApoE isoform modulates Ab-induced toxicity in cultured hippocampal pyramidal neurons. To the extent that homozygous expression of the e4 allele is associated with Ab release in vivo, such as in the most severe type of neurodegeneration in PCC (Vogt et al., 1998), ApoE genotype may be relevant to multifocal neuron losses in one group of cases.
There are a number of different ways to consider the risk liability of ApoE genotype. It has been suggested that the homozygous e4 individual has an eightfold greater risk of AD (Roses, 1995). Another statistical treatment suggests that this same genotype increases the risk by 10 years, with a single allele contributing one-half of this value. Another estimate is that the ApoE genetic locus accounts for approximately 50% of the attributable risk (Nalbantoglu et al., 1994). In contrast, a study of 310 families by Blacker et al. (1997) reported the greatest risk in early on-set cases and no role for e4 homozygotes between ages 60 and 65, suggesting greatest risk in early-onset cases and no role for transmission of a single e4 allele. Attempts to directly link ApoE genotype and AD neuropathology have shown only an increase in SP in e4 homozygotes, and the e4 risk liability is not limited to AD but includes critical coronary heart disease, hypertension, and epilepsy, as discussed below. The crucial question in relation to AD is, what other genes are responsible for focal neocortical pathology?
The essential argument for genetic subtypes and preliminary indications of their neuropathological impact arise from the interaction of different ApoE alleles on other genetic systems. While homozygous expression of the e4 allele increases the risk for AD, it exerts differential modulatory effects on other genes. The e4 allele decreases the age at onset for early-onset AD associated with APP mutations (Houlden et al., 1993), whereas the e4 allele may not have the same effect on the early-onset disease associated with presenilin mutations (Van Broeckhoven et al., 1994). The same gene, therefore, exerts different effects depending on the genetic environment. Conversely, the e2 allele may be protective against AD and so this allele is underrepresented in AD populations (Talbot et al., 1994; Corder et al., 1994). There are a number of plausible molecular explanations for this finding based on binding of the tau protein in NFT and interactions with Ab. However, this protective effect is absent in early-onset AB (Maestre et al., 1995) and in black populations (van Duijn et al., 1994). Thus, expression of the ApoE alleles influences the onset and progression of AD via their interactions with the products of other genes. For example, the e4 allele is associated with higher densities of SP in AD (Schmechel et al., 1993; Rebeck et al., 1993), and these SP have greater amounts of Ab40 which is not fibrillogenic (Gearing et al., 1996). However, PS1 mutations elevate the longer form of Ab (Borchelt et al., 1996; Scheuner et al., 1996), suggesting that homozygous expression of the ApoE e4 allele and a PS1 mutation will increase SP densities still further.
Studies of PCC suggest that, although homozygous expression of the ApoE e4 allele is associated with greater numbers of SP, the e4 risk is associated only with the most severe neurodegeneration and does not account for most laminar patterns in degeneration (Vogt et al., 1998). Because the ApoE risk is associated with altered processing of the APP throughout the body, including cortical deposition of SP{ in non-demented individuals with critical coronary heart disease and hypertension (Sparks et al., 1996), and in epileptogenic cortex (Gouras et al., 1997), the significance of the ApoE risk may be its influence on lipid metabolism. The issue raised by genomic studies of the ApoE is to what extent other genes may be responsible for neocortical pathology including disease subtypes and how the functions of these unknown genes are altered in e4 homozygotes. For example, Jordan et al. (1998) provide evidence that ApoE isoform modulates Ab-induced toxicity in cultured hippocampal pyramidal neurons. To the extent that homozygous expression of the e4 allele is associated with Ab release in vivo, such as in the most severe type of neurodegeneration in PCC (Vogt et al., 1998), ApoE genotype may be relevant to multifocal neuron losses in one group of cases.