Pain Processing, Cingulate Cortex and the Medical Pain System
by Brent A. Vogt, Ph.D.
President, Cingulum NeuroSciences Institute
Professor, Neuroscience and Physiology, SUNY Upstate Medical University
President, Cingulum NeuroSciences Institute
Professor, Neuroscience and Physiology, SUNY Upstate Medical University
Introduction
Our primary goal is to localize the domains of pain processing subserved by cingulate cortex in the context of the four-region neurobiological model presented in Functional Correlates. We will also identify the sources and features of nociceptive information, the place of cingulate cortex in CNS pain systems, and how this information is used in cingulate cortex to achieve behavioral goals. First, however, we need to consider the context and logic that underpins studies of the conscious experience of pain. An early theory of pain localization in the CNS was the gate control theory (Melzack and Wall, 1965). A goal of this theory was to provide mechanisms whereby one differentiates between innocuous and noxious stimulation. Inherent to this undertaking was the effort to explain two cognitive domains of pain processing; sensory-discriminative and affective-motivational. The concept of a duality in CNS pain processing was amplified by Albe-Fessard et al. (1985) who suggested these domains are differentially localized in the thalamus. The lateral nuclei were thought to mediate sensory-discrimination and the medial nuclei the affective-motivational component of processing. Although it is likely these functions are subserved in part by the two thalamic divisions as proposed, accounting for multiple aspects of the conscious experience of pain with thalamic mechanisms alone is difficult, particularly in light of the prominent projections of the medial and lateral thalamic nuclei to different parts of the cerebral cortex and the role of the cortex in anticipation and memory of many events including those associated with painful experiences. Indeed, one of the main functions of cortical pain processing is integrating the pain experience with other cortical information processing functions. A complete model of the medial and lateral systems, therefore, requires consideration of many cortical areas that are involved in such functions in addition to thalamic sites.
It has long been known that ablations of cingulate cortex (cingulotomy lesions) or its underlying white matter (cingulumotomy lesions) alleviate pain in chronic cancer patients (Foltz and White, 1962, 1968; Ballantine et al., 1967). It was also known that the nociceptive midline and intralaminar thalamic nuclei project to anterior cingulate cortex (ACC) (Vogt et al., 1979). Thus, it was unlikely that any domain for the cognitive processing of pain would be limited to the thalamus. In Neurobiology of Cingulate Cortex and Limbic Thalamus (Vogt et al., 1993), it was proposed that projections of the midline and intralaminar thalamic nuclei to ACC provided a cortical substrate for the affective-motivational aspects of pain processing. The concept of a duality in the contributions of cingulate cortex to pain processing was later extended such that the affective component is processed mainly in perigenual anterior cingulate cortex (pACC), while the motivational aspects related to motor functions are processed in midcingulate cortex (MCC; Vogt et al., 1996).
Although it is possible that a single cortical area may contribute to a single domain of pain processing, this does not imply that pain processing is the only function of any single cortical area. Indeed, no cortical area is engaged only in pain processing as a “pain center” in the pure sense. Even lamina I of the spinal cord processes the features of innocuous temperatures. A less restrictive view of a “pain center” implies that such a center is primarily, though not exclusively, involved in pain processing. In this context, lamina I of the spinal cord, and the posterior ventromedial and submedial nuclei in the thalamus are pain centers. Furthermore, based on the very large human functional imaging literature, it can be argued that no cortical area is involved primarily in pain processing and no cortical area is a pain center even under the less restrictive definition. Thus, as we look to the functions of cingulate cortex, we seek a functional context in which pain activity is employed to a particular end rather than where pain processing itself is the dominant function.
Finally, it is likely that each psychological domain of pain processing requires a network of structures and no single cortical area operates in isolation. The following localization approach to pain assessment in the mammalian cingulate gyrus does not suggest that each area of cingulate cortex is singularly involved in any one function. Rather, each area contributes to a network that is involved in the sensation, anticipation, responses, and storage in relation to noxious stimuli.
It has long been known that ablations of cingulate cortex (cingulotomy lesions) or its underlying white matter (cingulumotomy lesions) alleviate pain in chronic cancer patients (Foltz and White, 1962, 1968; Ballantine et al., 1967). It was also known that the nociceptive midline and intralaminar thalamic nuclei project to anterior cingulate cortex (ACC) (Vogt et al., 1979). Thus, it was unlikely that any domain for the cognitive processing of pain would be limited to the thalamus. In Neurobiology of Cingulate Cortex and Limbic Thalamus (Vogt et al., 1993), it was proposed that projections of the midline and intralaminar thalamic nuclei to ACC provided a cortical substrate for the affective-motivational aspects of pain processing. The concept of a duality in the contributions of cingulate cortex to pain processing was later extended such that the affective component is processed mainly in perigenual anterior cingulate cortex (pACC), while the motivational aspects related to motor functions are processed in midcingulate cortex (MCC; Vogt et al., 1996).
Although it is possible that a single cortical area may contribute to a single domain of pain processing, this does not imply that pain processing is the only function of any single cortical area. Indeed, no cortical area is engaged only in pain processing as a “pain center” in the pure sense. Even lamina I of the spinal cord processes the features of innocuous temperatures. A less restrictive view of a “pain center” implies that such a center is primarily, though not exclusively, involved in pain processing. In this context, lamina I of the spinal cord, and the posterior ventromedial and submedial nuclei in the thalamus are pain centers. Furthermore, based on the very large human functional imaging literature, it can be argued that no cortical area is involved primarily in pain processing and no cortical area is a pain center even under the less restrictive definition. Thus, as we look to the functions of cingulate cortex, we seek a functional context in which pain activity is employed to a particular end rather than where pain processing itself is the dominant function.
Finally, it is likely that each psychological domain of pain processing requires a network of structures and no single cortical area operates in isolation. The following localization approach to pain assessment in the mammalian cingulate gyrus does not suggest that each area of cingulate cortex is singularly involved in any one function. Rather, each area contributes to a network that is involved in the sensation, anticipation, responses, and storage in relation to noxious stimuli.
Concept of a Dual Pain System
The processing of sensory afferents in the cerebral cortex involves divergent processing of components of each sensory space. In the visual system, for example, there are separate and sequential, corticocortical projections for the analysis of form, color, movement and depth (DeYoe and Van Essen, 1988; Livingstone and Hubel, 1988; Zeki and Shipp, 1988). A divergence of functional processing of different features of nociceptor-evoked activity also occurs in the cerebral cortex with one domain involved in localization and sensory discrimination and the other involved in affective responses to noxious stimuli (e.g., Melzack and Casey, 1968; Melzack, 1975; Kenshalo and Willis, 1991). Numerous electrophysiological studies have demonstrated that the ventrobasal complex and somatosensory cortex contain neurons that are involved in the localization and intensity coding of noxious stimuli. However, little is known about regions that are involved in the affective and motivational responses to these stimuli. In addition, it is not known how these latter regions contribute to the perception of painful stimuli, learning associated with the prediction and avoidance of such stimuli or their role in chronic pain syndromes.
Please realize that the dual pain system concept is at odds with the neuromatrix concept. The dual system indicates that each circuit contributes something different to pain processing and the unifying principle is the mental perception. The neuromatrix, in contrast, is a group of interconnected systems that together produce a common output. For example, intensity coding in the neuromatrix is a function of all participants. In the dual system model, one may observe an intensity code in both systems, but this code is used to accomplish different goals in each. In the lateral system it is for coding the intensity of the noxious stimulus, while in the medial system it triggers autonomic outputs in one place (pACC) and modifies the reward properties of particular behaviors in another (MCC). Finally, to the extent that the two systems interact at the cortical level, their unique contributions to pain processing are blurred. In retrospect, this is why it is important that the medial and lateral systems have very few interconnections and do not participate in a matrix of equipotent structures.
Restricted stroke and neurosurgical cases support the notion there are at least two systems involved in aspects of pain processing and their associated cortices in different domains of the conscious experience of pain. A patient with a postcentral gyrus stroke involving mainly somatosensory cortex showed altered pain localization without changes in pain affect (Ploner et al., 1999). In contrast, cingulotomy or cingulumotomy lesions abolish a patient’s affective responses to a chronic pain yet leave intact their ability to localize the offending noxious stimulus (Ballantine et al., 1967). Thus, primary somatosensory and anterior cingulate cortices play different roles in pain processing.
The medial pain system is a theoretical construct that was introduced as a means of evaluating the role of cingulate cortex and some of its thalamic afferents in affective and motivational responses to noxious stimuli. It has been expanded to include nociceptive neurons in the spinal cord that project to the periaqueductal gray and the medial and intralaminar thalamic nuclei, the latter of which provide a source of nociceptive afferents to cingulate cortex. It also includes projections of ACC to the medial thalamus and periaqueductal gray that may mediate reflex responses to noxious stimuli. Finally, to account for avoidance learning processes, critical limbic connections of cingulate cortex are engaged including those of the amygdala.
Definition of the medial pain system depends on two neuroanatomical facts. First, all projections from the spinal cord do not terminate in the ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei. There is a second group of nuclei in the midline and intralaminar parts of the thalamus that receive spinal inputs. Second, these latter nuclei project to limbic cortical areas including cingulate cortex. Indeed, the medial pain system can be specifically identified where spinothalamic projections diverge in the thalamus. Thus, the medial system includes the medial and intralaminar thalamic nuclei (MITN) and their projections to pACC and MCC, which are together termed ACC. This system includes the connections of these areas with the periaqueductal gray that subserve responses to noxious stimuli and cholinergic connections that are involved, at least in part, in avoidance learning. Obviously this system is not independent in the CNS and the thalamic and periaqueductal gray nuclei are involved in other aspects of pain sensation and aversive responses to noxious stimuli. Finally, the emphasis on cingulate cortex in this analysis does not preclude other telencephalic structures from the medial pain system. Orbitofrontal area VLO, precentral agranular motor cortex, the amygdala and the anterior insula are also involved and may participate with cingulate cortex in the medial system in the affective and motivational responses to noxious stimuli. We begin with a brief review of the “lateral pain system” so the unique features of the medial pain system, and cingulate cortex therein, can be appreciated.
Please realize that the dual pain system concept is at odds with the neuromatrix concept. The dual system indicates that each circuit contributes something different to pain processing and the unifying principle is the mental perception. The neuromatrix, in contrast, is a group of interconnected systems that together produce a common output. For example, intensity coding in the neuromatrix is a function of all participants. In the dual system model, one may observe an intensity code in both systems, but this code is used to accomplish different goals in each. In the lateral system it is for coding the intensity of the noxious stimulus, while in the medial system it triggers autonomic outputs in one place (pACC) and modifies the reward properties of particular behaviors in another (MCC). Finally, to the extent that the two systems interact at the cortical level, their unique contributions to pain processing are blurred. In retrospect, this is why it is important that the medial and lateral systems have very few interconnections and do not participate in a matrix of equipotent structures.
Restricted stroke and neurosurgical cases support the notion there are at least two systems involved in aspects of pain processing and their associated cortices in different domains of the conscious experience of pain. A patient with a postcentral gyrus stroke involving mainly somatosensory cortex showed altered pain localization without changes in pain affect (Ploner et al., 1999). In contrast, cingulotomy or cingulumotomy lesions abolish a patient’s affective responses to a chronic pain yet leave intact their ability to localize the offending noxious stimulus (Ballantine et al., 1967). Thus, primary somatosensory and anterior cingulate cortices play different roles in pain processing.
The medial pain system is a theoretical construct that was introduced as a means of evaluating the role of cingulate cortex and some of its thalamic afferents in affective and motivational responses to noxious stimuli. It has been expanded to include nociceptive neurons in the spinal cord that project to the periaqueductal gray and the medial and intralaminar thalamic nuclei, the latter of which provide a source of nociceptive afferents to cingulate cortex. It also includes projections of ACC to the medial thalamus and periaqueductal gray that may mediate reflex responses to noxious stimuli. Finally, to account for avoidance learning processes, critical limbic connections of cingulate cortex are engaged including those of the amygdala.
Definition of the medial pain system depends on two neuroanatomical facts. First, all projections from the spinal cord do not terminate in the ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei. There is a second group of nuclei in the midline and intralaminar parts of the thalamus that receive spinal inputs. Second, these latter nuclei project to limbic cortical areas including cingulate cortex. Indeed, the medial pain system can be specifically identified where spinothalamic projections diverge in the thalamus. Thus, the medial system includes the medial and intralaminar thalamic nuclei (MITN) and their projections to pACC and MCC, which are together termed ACC. This system includes the connections of these areas with the periaqueductal gray that subserve responses to noxious stimuli and cholinergic connections that are involved, at least in part, in avoidance learning. Obviously this system is not independent in the CNS and the thalamic and periaqueductal gray nuclei are involved in other aspects of pain sensation and aversive responses to noxious stimuli. Finally, the emphasis on cingulate cortex in this analysis does not preclude other telencephalic structures from the medial pain system. Orbitofrontal area VLO, precentral agranular motor cortex, the amygdala and the anterior insula are also involved and may participate with cingulate cortex in the medial system in the affective and motivational responses to noxious stimuli. We begin with a brief review of the “lateral pain system” so the unique features of the medial pain system, and cingulate cortex therein, can be appreciated.
Lateral Pain System
Some peripheral somatic sensory receptors have high thresholds of activation and respond to levels of mechanical and/or thermal stimulation that are perceived to be noxious (Perl, 1987; Willis, 1985). These include mechanical stimuli applied with a von Frey hair of 4-11 g/mm2 and thermal stimuli over 45°C (Hardy, 1953). These nociceptors have axons that conduct in the A* and C range and they have larger receptive fields than those of other somatic receptors, the former being 15 to 100 mm2 (Perl, 1968). Nociceptor projections to the spinal cord terminate mainly in Rexed's laminae I, II and V (Light and Perl, 1979; Craig et al., 1988) and in lamina I/II in the nucleus caudalis of the trigeminal complex (Hayashi, 1985; Shigenaga et al., 1986). Therefore, it is in these layers that neurons are located that code the properties of noxious stimulation (Kumazawa and Perl, 1978; Mosso and Kruger, 1973).
The VPL and VPM thalamic nuclei are the principal relay nuclei for nociceptor-derived information from spinal cord and trigeminal complex neurons, respectively, to somatosensory cortex. Therefore, these nuclei are key intermediates in the lateral pain system. The main projection to the VPL originates in spinal laminae I and V (Willis et al., 1979; Craig et al., 1989), although neurons in layers VI, VII and VIII are labeled in smaller numbers following injections of retrograde tracers into the VPL. Spinothalamic projection neurons have been characterized (Applebaum et al., 1975; Willis et al., 1975; Giesler et al., 1981; Craig and Kniffki, 1985; Willis, 1985; Craig and Hunsley,1991) as 1) nociceptive-specific units that respond only to high threshold mechanical or thermal stimulation, 2) wide dynamic range units that respond to both noxious and innocuous stimuli, 3) multireceptive neurons responding to heat, pinch, and cold, and 4) thermoreceptive-specific neurons for cold stimuli. Although the wide-dynamic range neurons are located mainly in the deep laminae of the spinal cord, the other three classes are mainly in lamina I. Finally, the receptive fields of spinothalamic projection neurons are nearly always contralateral to the stimulus, somatotopically organized and are considerably larger than those for nociceptors themselves. Thus, receptive field organization and stimulus coding are preserved by afferents to the VPL and VPM.
The response characteristics of neurons in the VPL are similar to those of spinothalamic projection neurons. Both nociceptive-specific and wide dynamic range neurons have been identified in several species. The majority of these neurons code stimulus intensity via their frequency and/or recruitment. In a comprehensive study of the responses of VPL neurons in the monkey, Kenshalo et al. (1980) showed polymodal responses to noxious mechanical and thermal stimulation in 81% of units tested. Of 54 units tested, 48 had responses with wide dynamic range properties and only six had nociceptive-specific responses. Both types of neurons had small contralateral receptive fields and their responses adapted to frequent applications of noxious thermal stimuli. The properties of these neurons are likely generated in the spinal cord, since lesions of the ventrolateral spinal cord white matter abolished them. Also, their responses are characteristic of thalamocortical projection neurons because many of these units could be antidromically activated with electrical stimulation of somatosensory areas 1 and 3b.
Primary somatosensory cortex is involved in pain processing. Ablations of primary somatosensory cortex in the monkey impair discrimination among noxious thermal stimuli without altering the detection of these stimuli (Kenshalo et al., 1991). Electrophysiological studies have shown that neurons in primary and secondary somatosensory cortices have responses that correlate with the duration of noxious stimulation. Nearly all nociceptive neurons in the monkey somatosensory cortex respond to both innocuous and noxious stimuli; i.e., 84% are polymodal (Kenshalo and Isensee, 1983). Excitatory responses are common and the majority have small receptive fields confined to one contralateral limb. In about 20% of the neurons, a small contralateral receptive field to innocuous stimuli could be defined, but the units would also respond to noxious stimulation anywhere on the body surface. Most nociceptive neurons in monkey primary somatosensory cortex code for stimulus intensity (Kenshalo et al., 1988; Chudler et al., 1990). The threshold for noxious thermal stimuli is 44.9° C when interstimulus intervals of 180 sec and somewhat higher when the interval is 30 sec. Many of these neurons show little adaptation to continued high levels of noxious heat (49° C) in contrast to spinothalamic and ventral thalamic neurons that show marked adaptation (Kenshalo et al., 1980; Giesler et al., 1981).
Ploner et al. (1999) showed that a stoke in human primary somatosensory cortex greatly reduces localization of noxious activity without interfering with affective responses thereto. This finding is quite important because it not only validates a role for somatosensory cortex in nociception but it also suggests that other regions, such as ACC, are involved in other cognitive domains of pain processing.
Secondary somatosensory cortex is also involved in pain processing. Recruitment of A* fibers with high intensity electrical stimulation of the tooth pulp evokes a morphine-sensitive, long latency potential in this cortex (Chundler et al., 1986) and the amplitude of the N3-P3 potential correlates highly with the probability of escape behavior. Responses of a small number of neurons in secondary somatosensory cortex specific for noxious stimuli have also been described (Dong et al., 1989). These units have large and often bilateral receptive fields, and only weak coding for the intensity of graded mechanical stimulation and they show little adaptation during prolonged stimulation and efficiently code the duration of the stimulus. Finally, all nociceptive neurons in this cortex nociceptive-specific, i.e., no neurons have wide dynamic range properties.
In conclusion, definition of the lateral and medial pain systems depends upon the divergence of spinothalamic projections in the posterior part of the thalamus. The lateral system has spinothalamic projections to the VPL and trigeminothalamic projections to VPM thalamic nuclei. These nuclei project in turn to primary and secondary somatosensory cortices and transmit information about the intensity, duration and location of noxious stimuli with few alterations in coding parameters between the spinal cord and cortex. Such a system is ideal for providing detailed information about the somatic location and characteristics of particular noxious stimuli. In contrast, the medial pain system is composed of spinothalamic projections to medial thalamic nuclei and from there to limbic cortices including the ACC. Nociceptive neurons in this system have little or no somatotopic organization and are best suited for processes associated with affective responses to noxious stimuli, i.e., detection and avoidance processes, since affective responses do not depend on the somatic location of a stimulus.
The VPL and VPM thalamic nuclei are the principal relay nuclei for nociceptor-derived information from spinal cord and trigeminal complex neurons, respectively, to somatosensory cortex. Therefore, these nuclei are key intermediates in the lateral pain system. The main projection to the VPL originates in spinal laminae I and V (Willis et al., 1979; Craig et al., 1989), although neurons in layers VI, VII and VIII are labeled in smaller numbers following injections of retrograde tracers into the VPL. Spinothalamic projection neurons have been characterized (Applebaum et al., 1975; Willis et al., 1975; Giesler et al., 1981; Craig and Kniffki, 1985; Willis, 1985; Craig and Hunsley,1991) as 1) nociceptive-specific units that respond only to high threshold mechanical or thermal stimulation, 2) wide dynamic range units that respond to both noxious and innocuous stimuli, 3) multireceptive neurons responding to heat, pinch, and cold, and 4) thermoreceptive-specific neurons for cold stimuli. Although the wide-dynamic range neurons are located mainly in the deep laminae of the spinal cord, the other three classes are mainly in lamina I. Finally, the receptive fields of spinothalamic projection neurons are nearly always contralateral to the stimulus, somatotopically organized and are considerably larger than those for nociceptors themselves. Thus, receptive field organization and stimulus coding are preserved by afferents to the VPL and VPM.
The response characteristics of neurons in the VPL are similar to those of spinothalamic projection neurons. Both nociceptive-specific and wide dynamic range neurons have been identified in several species. The majority of these neurons code stimulus intensity via their frequency and/or recruitment. In a comprehensive study of the responses of VPL neurons in the monkey, Kenshalo et al. (1980) showed polymodal responses to noxious mechanical and thermal stimulation in 81% of units tested. Of 54 units tested, 48 had responses with wide dynamic range properties and only six had nociceptive-specific responses. Both types of neurons had small contralateral receptive fields and their responses adapted to frequent applications of noxious thermal stimuli. The properties of these neurons are likely generated in the spinal cord, since lesions of the ventrolateral spinal cord white matter abolished them. Also, their responses are characteristic of thalamocortical projection neurons because many of these units could be antidromically activated with electrical stimulation of somatosensory areas 1 and 3b.
Primary somatosensory cortex is involved in pain processing. Ablations of primary somatosensory cortex in the monkey impair discrimination among noxious thermal stimuli without altering the detection of these stimuli (Kenshalo et al., 1991). Electrophysiological studies have shown that neurons in primary and secondary somatosensory cortices have responses that correlate with the duration of noxious stimulation. Nearly all nociceptive neurons in the monkey somatosensory cortex respond to both innocuous and noxious stimuli; i.e., 84% are polymodal (Kenshalo and Isensee, 1983). Excitatory responses are common and the majority have small receptive fields confined to one contralateral limb. In about 20% of the neurons, a small contralateral receptive field to innocuous stimuli could be defined, but the units would also respond to noxious stimulation anywhere on the body surface. Most nociceptive neurons in monkey primary somatosensory cortex code for stimulus intensity (Kenshalo et al., 1988; Chudler et al., 1990). The threshold for noxious thermal stimuli is 44.9° C when interstimulus intervals of 180 sec and somewhat higher when the interval is 30 sec. Many of these neurons show little adaptation to continued high levels of noxious heat (49° C) in contrast to spinothalamic and ventral thalamic neurons that show marked adaptation (Kenshalo et al., 1980; Giesler et al., 1981).
Ploner et al. (1999) showed that a stoke in human primary somatosensory cortex greatly reduces localization of noxious activity without interfering with affective responses thereto. This finding is quite important because it not only validates a role for somatosensory cortex in nociception but it also suggests that other regions, such as ACC, are involved in other cognitive domains of pain processing.
Secondary somatosensory cortex is also involved in pain processing. Recruitment of A* fibers with high intensity electrical stimulation of the tooth pulp evokes a morphine-sensitive, long latency potential in this cortex (Chundler et al., 1986) and the amplitude of the N3-P3 potential correlates highly with the probability of escape behavior. Responses of a small number of neurons in secondary somatosensory cortex specific for noxious stimuli have also been described (Dong et al., 1989). These units have large and often bilateral receptive fields, and only weak coding for the intensity of graded mechanical stimulation and they show little adaptation during prolonged stimulation and efficiently code the duration of the stimulus. Finally, all nociceptive neurons in this cortex nociceptive-specific, i.e., no neurons have wide dynamic range properties.
In conclusion, definition of the lateral and medial pain systems depends upon the divergence of spinothalamic projections in the posterior part of the thalamus. The lateral system has spinothalamic projections to the VPL and trigeminothalamic projections to VPM thalamic nuclei. These nuclei project in turn to primary and secondary somatosensory cortices and transmit information about the intensity, duration and location of noxious stimuli with few alterations in coding parameters between the spinal cord and cortex. Such a system is ideal for providing detailed information about the somatic location and characteristics of particular noxious stimuli. In contrast, the medial pain system is composed of spinothalamic projections to medial thalamic nuclei and from there to limbic cortices including the ACC. Nociceptive neurons in this system have little or no somatotopic organization and are best suited for processes associated with affective responses to noxious stimuli, i.e., detection and avoidance processes, since affective responses do not depend on the somatic location of a stimulus.
Midline and Intralaminar Thalamic Nuclei
The MITN form a complex of about 20 cytoarchitecturally and chemically unique nuclei interspersed among the “specific” thalamic nuclei. Inputs from the medullary cholinergic activating systems and presumed diffuse projections of the MITN to the cerebral cortex led to the early view that the MITN are part of a diffuse system for arousal. However, inputs to the MITN are not nonspecific, although some may be associated with arousal, they do not have nonspecific response properties, and they do not project diffusely throughout the cerebral cortex. For example, about 50% of neurons in the dorsocaudal part of the central lateral (Cl) and laterodorsal (LD) nuclei respond to orbital position of the eye and others respond to aspects of saccadic eye movements (Schlaug-Rey and Schlaug, 1984). These latter investigators concluded, “Although widespread, the internal medullary lamina connections are not general, and, although complex, the activity of IML cells appears much more specific then originally suspected. If anything, the units described in this report were characterized by the individuality of the firing patterns among neighboring cells. This is exactly the reverse of what one would expect in a mass activation.”
Our view is that some MITN play a role in pain processing via projections to cingulate cortex and these may be some of the specific thalamic nuclei for pACC and MCC. The expectation that they are not specific for ACC was based on the view that the anterior thalamic nuclei were the specific nuclei for cingulate cortex. Although the anterior dorsal and anterior ventral thalamic nuclei are specific for monkey retrosplenial cortex (Vogt et al., 1987), the anteromedial nucleus (AM) projects throughout the entire cingulate gyrus. The AM has a substantial projection to ACC in rabbit (Vogt et al., 1993, Figure 10.2), however, the ventral anterior and parvicellular medial dorsal nuclei make projections of equivalent strength (i.e., number of retrogradely labeled neurons) to ACC and it is not specific in the sense that it is a unique feature of ACC. Thus, AM, MDp, VA and some of the MITN may be the specific nuclei for the ACC. In addition, most of the MITN that project to anterior cortex do not project posteriorally; only the central superior lateral (Csl) nucleus projects significantly to both regions. Retrograde labeling following horseradish peroxidase (HRP) injections into ACC occurs in the following nuclei in addition to Csl: central densocellular (Cdc), central latocellular (Clc), central lateral (Cl), reunions (Re), central inferior (Cif), central superior (Cs), paraventricular (Pv), parataenial (Pt), parafascicular (Pf), and limitans (Li). When speaking of the specific thalamic inputs to ACC (i.e., both pACC and MCC), these nuclei must be considered.
Our view is that some MITN play a role in pain processing via projections to cingulate cortex and these may be some of the specific thalamic nuclei for pACC and MCC. The expectation that they are not specific for ACC was based on the view that the anterior thalamic nuclei were the specific nuclei for cingulate cortex. Although the anterior dorsal and anterior ventral thalamic nuclei are specific for monkey retrosplenial cortex (Vogt et al., 1987), the anteromedial nucleus (AM) projects throughout the entire cingulate gyrus. The AM has a substantial projection to ACC in rabbit (Vogt et al., 1993, Figure 10.2), however, the ventral anterior and parvicellular medial dorsal nuclei make projections of equivalent strength (i.e., number of retrogradely labeled neurons) to ACC and it is not specific in the sense that it is a unique feature of ACC. Thus, AM, MDp, VA and some of the MITN may be the specific nuclei for the ACC. In addition, most of the MITN that project to anterior cortex do not project posteriorally; only the central superior lateral (Csl) nucleus projects significantly to both regions. Retrograde labeling following horseradish peroxidase (HRP) injections into ACC occurs in the following nuclei in addition to Csl: central densocellular (Cdc), central latocellular (Clc), central lateral (Cl), reunions (Re), central inferior (Cif), central superior (Cs), paraventricular (Pv), parataenial (Pt), parafascicular (Pf), and limitans (Li). When speaking of the specific thalamic inputs to ACC (i.e., both pACC and MCC), these nuclei must be considered.
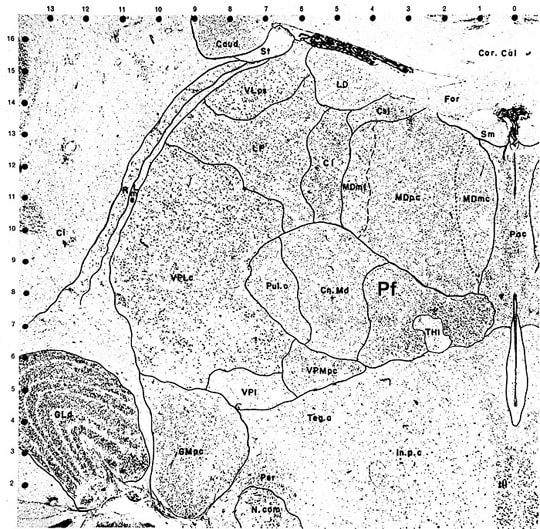
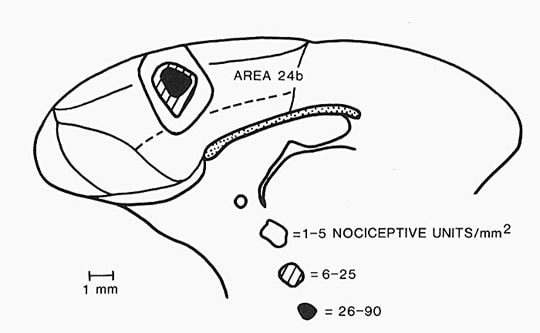
Since a complete description of the MITN organization is too complex for the current task, let us consider just one of these nuclei as a model of how this system is structured. The parafascicular nucleus (Pf) receives spinothalamic afferents, contains nociceptive neurons, projects to ACC and transmits nociceptive information thereto. Figure 1 is plate #68 by Olszewski (1952) and it shows a Nissl preparation of the posterior thalamus with Pf surrounding much of the habenulointerpeduncular tract (THI). The Pf is densely packed with medium-to-large sized neurons and is medial to the centrum medianum nucleus. Just caudal to Pf lies the limitans nucleus that also receives STT inputs, contains nociceptive neurons, and has substantial ACC projections.
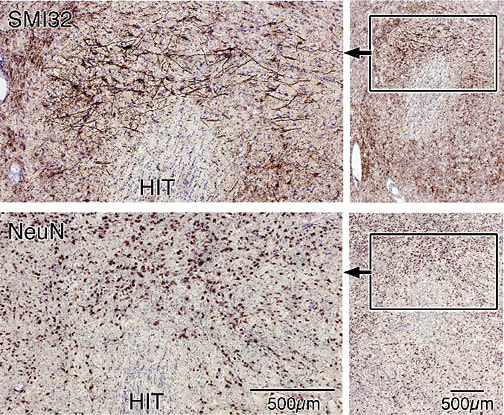
Figure 2 shows the Pf nucleus immunoreacted for nonphosphorylated, intermediate neurofilaments (neurofilament proteins; NFP shown with the SMI32 antibody) and for neuron-specific nuclear binding protein (NeuN). Both sections were counterstained with thionin that renders the glial and endothelial cells blue. The photographs are taken at a level similar to that of Olszewski where Pf surrounds the dorsal part of the habenulointerpeduncular tract (HIT). The largest Pf neurons are dorsal to the HIT and are heavily reactive for NFP (Fig. 2, SMI32). A NeuN section emphasizes the presence of large neurons in the dorsal sector of this nucleus and their interdigitation with groups of smaller neurons. It is from the sector of large neurons dorsal to the HIT that axons originate for transit to the ACC.
Although anterior cingulate and insular cortices are the most frequently activated in acute studies of human pain processing (Casey, 1999; Peyron et al., 2000; Derbyshire, 2000), there are only weak interconnections between these areas (Mesulam and Mufson, 1982; Vogt and Pandya, 1987). We suggested, therefore, that the reason both regions are jointly activated by noxious stimuli is they both receive parallel projections from the MITN (Vogt and Sikes, 2000). In the context of pain processing, the MITN are of pivotal importance. Some MITN contain neurons that both project to ACC and are nociceptive. Casey (1966) and Dong et al. (1978) showed that neurons in the Pf, Cl, Li, and MD contain nociceptive neurons and a subset responded to innocuous stimulation (i.e., brief tap) and these properties have been demonstrated for neurons in ACC (Sikes and Vogt, 1992). These four nuclei stand out as particularly important to pain processing in ACC.
Rexed’s spinal cord laminae I and V-VIII contain neurons that project to the medial thalamus (Carstens and Trevino, 1978; Willis et al., 1979; Craig et al., 1989). Craig et al. (1989) reported that about 60% of the lamina I, VII and VIII spinothalamic projection neurons terminate in the medial thalamus, while around 40% of these neurons in laminae V and VI project to the medial thalamus. The medial thalamic projection neurons in lamina I are in the ventrolateral part of this layer in contrast to the lateral projection neurons that are in the dorsomedial part of lamina I. The submedial nucleus appears to be unique among these nuclei, since most of its input arises from lamina I in the cat (Craig and Burton, 1981), while in the rat most of this input originates in deep layers of the spinal cord (Dado and Giesler, 1990).
Craig (1994, 1995) published two review articles with illustrations of the thalamic termination sites of lamina I in monkey and labeled some nuclei previously shown to project to pACC/MCC including the Pf, Li, and VM (Vogt et al., 1987; Minciacchi et al., 1986). Apkarian and Hodge (1989) anterogradely labeled STT inputs with HRP in MDpc and MDdc, Cl, suprageniculate (SG), subfascicular, Pf, and the PCN nuclei. Earlier studies of large injections of HRP including lamina V labeled neurons in additional nuclei that also project to cingulate cortex including CeM, reuniens (Re), Pv, and paracentral (Pc) (Bovie, 1979; Mantyh, 1983). Expression of the immediate-early gene cfos following noxious peripheral nerve electrical stimulation labels neurons in Pf, Pt, Pv, Re, and the rhomboid (Rh) nuclei (Bullitt, 1990). Thus, there is a wealth of evidence that nociceptive, spinal projections terminate in thalamic nuclei that project in turn to ACC.
Receptive field properties. Neurons in the Pf, Cl, Li, Sm, and MD nuclei respond to noxious stimuli (Casey, 1966; Dong et al., 1978; Peschanski et al., 1981; Miletic and Coffield, 1989; Craig, 1990). These stimuli include intense pressures such as pinches to the skin with serrated forceps and temperatures over 43°C. Between 75% and 91% of these responses are excitatory and they can be brief (20-100 msec) or involve prolonged after discharges (2-30 sec). The receptive fields are very large including one side of the body or the entire body surface. Most studies report limited responses of medial thalamic neurons to innocuous stimuli. The only effective stimuli are very brief taps to the skin, and many nociceptive neurons respond to tap (Dong et al., 1978). Finally, there are reports of intensity coding for noxious stimuli in the monkey medial thalamus. One reported that neurons in the medial nuclei of anesthetized monkeys code for the intensity of noxious stimuli (Dong et al., 1978) and another showed that intensity coding can occur in the medial thalamus of the alert monkey (Bushnell and Duncan, 1989). In addition to these findings in experimental animals, there is evidence that neurons in the human Pf nucleus respond to noxious stimuli. They are primarily excitatory and the receptive fields are large and usually bilateral (Ishijima et al., 1975), however, 4 of the 7 cases were being treated for intractable pain and may not reflect normal, acute nociceptive responses.
Since the receptive field properties of medial thalamic neurons may dominate those of cingulate cortex, it is important to consider where the broad receptive field properties of neurons in the medial thalamic nuclei originate. There are two reasons for the limited somatotopic organization of receptive fields of medial thalamic nociceptive neurons. First, there is little or no segmental organization of spinal cord inputs to the medial nuclei. Only the projection to the submedial nucleus has a limited topographical organization. In this instance all spinal cord inputs terminate rostrally in the submedial nucleus and the nucleus caudalis of the trigeminal complex terminates caudally in the submedial nucleus (Craig and Burton, 1981). Second, some spinothalamic projection neurons have broad receptive field properties. Giesler et al. (1981) identified a population of neurons in laminae VI and VII of the spinal cord that have receptive fields that are not limited to individual dermatomes, and their axons have slow conduction rates (18.6 m/sec compared to 37.1 m/sec for neurons projecting to the lateral thalamus). Thus, convergence of segmental spinothalamic afferents and the broad receptive fields of some deep spinothalamic projection neurons may account for the very large receptive field properties observed for neurons in medial thalamic nuclei.
Thalamotomy and electrical stimulation for pain relief. Ablation of MITN can alter responses to noxious stimuli in experimental animals and relieve chronic pain in human patients. Kaelber et al. (1975) showed that lesions of the centrum medianum-parafascicular complex in cats abolish escape responses to tooth pulp stimulation. Neurosurgical observations indicate that lesions in the medial thalamus that include the Pf alleviate chronic pain in humans and this procedure is one avenue for producing analgesia surgically (Mark and Ervin, 1969; Hitchcock and Teixeira, 1980). One interpretation of these observations is that medial thalamic lesions either directly abolish conscious sensations associated with noxious afferents in the thalamus or they deafferent higher levels at which such sensations occur in the cerebral cortex. In light of the evidence directly implicating ACC in such responses, the latter possibility appears to be an important alternative.
Electrical stimulation of the medial thalamic nuclei may also reduce behavioral responses to noxious stimuli. Stimulation of the Pv nucleus suppresses scratching and biting in arthritic rats and prolongs reaction times in the tail-flick and hot-plate tests (Kupers et al., 1988). Richardson and Akil (1977a,b) report that electrical stimulation in the periventricular region of the thalamus, including Pf, alleviates chronic pain in human patients. These investigators recognized other electrical stimulation studies of this area that evoked diffuse burning pain rather than analgesia (Nashold et al., 1969). Richardson and Akil (1977a) attribute the analgesic effects of their stimulation to the lower stimulation currents in their patients. It might also be noted that Sano (1977) consistently evoked diffuse burning sensations in the contralateral half of the body or the entire body surface when stimulating in the medial thalamus. In some instances the spontaneous pain of these individuals was exaggerated by such stimulation. His stimulation sites, however, were mainly in the centrum medianum and to a lesser extent Pf. Thus, differences in responses to electrical stimulation may be due to both stimulation parameters and the thalamic sites stimulated.
Reciprocal medial thalamic-periaqueductal gray connections. The periaqueductal gray (PAG) is a key player in responses to noxious stimuli, particularly as a link in the descending noxious inhibitory system (La Bars et al., 1979; Craig, 1994). The PAG receives inputs from laminae I and V of the spinal cord (Liu, 1983); some of which are the collaterals of axons that project to medial thalamic nuclei (Liu, 1986; Harmann et al., 1988). It is well known that injection of opiates into the PAG (Yaksh et al., 1988; Carstens et al., 1990) and electrical stimulation of the PAG (Mayer and Liebeskind, 1974; Zhang et al., 1991) produces analgesia. Thus, it is likely that components of the medial pain system that are connected with the PAG may contribute to modulation of responses to nociceptor stimulation. Since the PAG has reciprocal connections with the medial thalamic nuclei and receives ACC input, the contribution of the PAG to functions of the medial pain system needs to be elaborated without detracting from its involvement in lateral pain system functions.
Anterograde (Mantyh, 1983) and retrograde (Comans and Snow, 1981) tracing studies showed that dorsal and ventral parts of the PAG have ascending projections to the centrolateral, centromedial, dorsal hypothalamic, reuniens, Pf, paracentral, and paraventricular nuclei. Furthermore, the activity of neurons in the Pf, mediodorsal, centromedial and centrolateral nuclei can be inhibited with electrical stimulation of the PAG and somatosensory cortex. Andersen (1986) showed that inhibitory postsynaptic potentials produced by electrical stimulation of PAG override excitatory responses in the thalamus evoked by nociceptor stimulation.
Injections of HRP into the PAG (Marchand and Hagino, 1983) demonstrated the reciprocal pathway between medial thalamic nuclei and the PAG. Labeled neurons following PAG injections were in the Pf and the dorsal premamillary and dorsomedial and ventromedial nuclei of the hypothalamus. Also, electrical stimulation of Pf increases local cerebral glucose utilization in the PAG and deep layers of the superior colliculus (Aiko et al., 1987). An electrophysiological study by Sakata et al. (1988) showed that 80% of the neurons in the PAG and adjacent reticular formation produced excitatory responses following electrical stimulation of Pf and 91% of neurons that responded to peripheral noxious stimuli were also driven by Pf stimulation. Thus, there are reciprocal connections between the medial thalamic nuclei and the PAG. While the descending projection is excitatory, the ascending projection from the PAG appears to be inhibitory. It is possible that the reciprocal connections between these areas modulate responses to noxious stimuli and pain perception at cortical levels.
Medial & intralaminar thalamic projections to ACC. The earliest evidence that ACC may be directly linked to the pain system arose from studies of the monkey that showed projections of the MITN were very prominent to anterior but not posterior cingulate cortex (Vogt et al., 1979, 1987). Work with an acute rabbit preparation for analysis of responses to noxious stimuli directs the attention of this discussion to the projections of thalamic nuclei in the rabbit that likely receive spinothalamic afferents and contain nociceptive neurons. As discussed above, these include the centrolateral, Pf, paraventricular, parataenial, reuniens, submedial and ventromedial nuclei. A rabbit case with a large HRP injection mainly into rostral area 24b is presented in Figure 3. In this case there is substantial labeling of neurons in the reuniens, submedial and ventromedial nuclei and moderate labeling in the centrolateral, Pf, and parataenial nuclei. The only nucleus that has been reported to receive spinothalamic afferents in other species and does not contain labeled neurons in this case is the paraventricular nucleus. Other MITN that contain labeled neurons are the central and rhomboid nuclei.
Although anterior cingulate and insular cortices are the most frequently activated in acute studies of human pain processing (Casey, 1999; Peyron et al., 2000; Derbyshire, 2000), there are only weak interconnections between these areas (Mesulam and Mufson, 1982; Vogt and Pandya, 1987). We suggested, therefore, that the reason both regions are jointly activated by noxious stimuli is they both receive parallel projections from the MITN (Vogt and Sikes, 2000). In the context of pain processing, the MITN are of pivotal importance. Some MITN contain neurons that both project to ACC and are nociceptive. Casey (1966) and Dong et al. (1978) showed that neurons in the Pf, Cl, Li, and MD contain nociceptive neurons and a subset responded to innocuous stimulation (i.e., brief tap) and these properties have been demonstrated for neurons in ACC (Sikes and Vogt, 1992). These four nuclei stand out as particularly important to pain processing in ACC.
Rexed’s spinal cord laminae I and V-VIII contain neurons that project to the medial thalamus (Carstens and Trevino, 1978; Willis et al., 1979; Craig et al., 1989). Craig et al. (1989) reported that about 60% of the lamina I, VII and VIII spinothalamic projection neurons terminate in the medial thalamus, while around 40% of these neurons in laminae V and VI project to the medial thalamus. The medial thalamic projection neurons in lamina I are in the ventrolateral part of this layer in contrast to the lateral projection neurons that are in the dorsomedial part of lamina I. The submedial nucleus appears to be unique among these nuclei, since most of its input arises from lamina I in the cat (Craig and Burton, 1981), while in the rat most of this input originates in deep layers of the spinal cord (Dado and Giesler, 1990).
Craig (1994, 1995) published two review articles with illustrations of the thalamic termination sites of lamina I in monkey and labeled some nuclei previously shown to project to pACC/MCC including the Pf, Li, and VM (Vogt et al., 1987; Minciacchi et al., 1986). Apkarian and Hodge (1989) anterogradely labeled STT inputs with HRP in MDpc and MDdc, Cl, suprageniculate (SG), subfascicular, Pf, and the PCN nuclei. Earlier studies of large injections of HRP including lamina V labeled neurons in additional nuclei that also project to cingulate cortex including CeM, reuniens (Re), Pv, and paracentral (Pc) (Bovie, 1979; Mantyh, 1983). Expression of the immediate-early gene cfos following noxious peripheral nerve electrical stimulation labels neurons in Pf, Pt, Pv, Re, and the rhomboid (Rh) nuclei (Bullitt, 1990). Thus, there is a wealth of evidence that nociceptive, spinal projections terminate in thalamic nuclei that project in turn to ACC.
Receptive field properties. Neurons in the Pf, Cl, Li, Sm, and MD nuclei respond to noxious stimuli (Casey, 1966; Dong et al., 1978; Peschanski et al., 1981; Miletic and Coffield, 1989; Craig, 1990). These stimuli include intense pressures such as pinches to the skin with serrated forceps and temperatures over 43°C. Between 75% and 91% of these responses are excitatory and they can be brief (20-100 msec) or involve prolonged after discharges (2-30 sec). The receptive fields are very large including one side of the body or the entire body surface. Most studies report limited responses of medial thalamic neurons to innocuous stimuli. The only effective stimuli are very brief taps to the skin, and many nociceptive neurons respond to tap (Dong et al., 1978). Finally, there are reports of intensity coding for noxious stimuli in the monkey medial thalamus. One reported that neurons in the medial nuclei of anesthetized monkeys code for the intensity of noxious stimuli (Dong et al., 1978) and another showed that intensity coding can occur in the medial thalamus of the alert monkey (Bushnell and Duncan, 1989). In addition to these findings in experimental animals, there is evidence that neurons in the human Pf nucleus respond to noxious stimuli. They are primarily excitatory and the receptive fields are large and usually bilateral (Ishijima et al., 1975), however, 4 of the 7 cases were being treated for intractable pain and may not reflect normal, acute nociceptive responses.
Since the receptive field properties of medial thalamic neurons may dominate those of cingulate cortex, it is important to consider where the broad receptive field properties of neurons in the medial thalamic nuclei originate. There are two reasons for the limited somatotopic organization of receptive fields of medial thalamic nociceptive neurons. First, there is little or no segmental organization of spinal cord inputs to the medial nuclei. Only the projection to the submedial nucleus has a limited topographical organization. In this instance all spinal cord inputs terminate rostrally in the submedial nucleus and the nucleus caudalis of the trigeminal complex terminates caudally in the submedial nucleus (Craig and Burton, 1981). Second, some spinothalamic projection neurons have broad receptive field properties. Giesler et al. (1981) identified a population of neurons in laminae VI and VII of the spinal cord that have receptive fields that are not limited to individual dermatomes, and their axons have slow conduction rates (18.6 m/sec compared to 37.1 m/sec for neurons projecting to the lateral thalamus). Thus, convergence of segmental spinothalamic afferents and the broad receptive fields of some deep spinothalamic projection neurons may account for the very large receptive field properties observed for neurons in medial thalamic nuclei.
Thalamotomy and electrical stimulation for pain relief. Ablation of MITN can alter responses to noxious stimuli in experimental animals and relieve chronic pain in human patients. Kaelber et al. (1975) showed that lesions of the centrum medianum-parafascicular complex in cats abolish escape responses to tooth pulp stimulation. Neurosurgical observations indicate that lesions in the medial thalamus that include the Pf alleviate chronic pain in humans and this procedure is one avenue for producing analgesia surgically (Mark and Ervin, 1969; Hitchcock and Teixeira, 1980). One interpretation of these observations is that medial thalamic lesions either directly abolish conscious sensations associated with noxious afferents in the thalamus or they deafferent higher levels at which such sensations occur in the cerebral cortex. In light of the evidence directly implicating ACC in such responses, the latter possibility appears to be an important alternative.
Electrical stimulation of the medial thalamic nuclei may also reduce behavioral responses to noxious stimuli. Stimulation of the Pv nucleus suppresses scratching and biting in arthritic rats and prolongs reaction times in the tail-flick and hot-plate tests (Kupers et al., 1988). Richardson and Akil (1977a,b) report that electrical stimulation in the periventricular region of the thalamus, including Pf, alleviates chronic pain in human patients. These investigators recognized other electrical stimulation studies of this area that evoked diffuse burning pain rather than analgesia (Nashold et al., 1969). Richardson and Akil (1977a) attribute the analgesic effects of their stimulation to the lower stimulation currents in their patients. It might also be noted that Sano (1977) consistently evoked diffuse burning sensations in the contralateral half of the body or the entire body surface when stimulating in the medial thalamus. In some instances the spontaneous pain of these individuals was exaggerated by such stimulation. His stimulation sites, however, were mainly in the centrum medianum and to a lesser extent Pf. Thus, differences in responses to electrical stimulation may be due to both stimulation parameters and the thalamic sites stimulated.
Reciprocal medial thalamic-periaqueductal gray connections. The periaqueductal gray (PAG) is a key player in responses to noxious stimuli, particularly as a link in the descending noxious inhibitory system (La Bars et al., 1979; Craig, 1994). The PAG receives inputs from laminae I and V of the spinal cord (Liu, 1983); some of which are the collaterals of axons that project to medial thalamic nuclei (Liu, 1986; Harmann et al., 1988). It is well known that injection of opiates into the PAG (Yaksh et al., 1988; Carstens et al., 1990) and electrical stimulation of the PAG (Mayer and Liebeskind, 1974; Zhang et al., 1991) produces analgesia. Thus, it is likely that components of the medial pain system that are connected with the PAG may contribute to modulation of responses to nociceptor stimulation. Since the PAG has reciprocal connections with the medial thalamic nuclei and receives ACC input, the contribution of the PAG to functions of the medial pain system needs to be elaborated without detracting from its involvement in lateral pain system functions.
Anterograde (Mantyh, 1983) and retrograde (Comans and Snow, 1981) tracing studies showed that dorsal and ventral parts of the PAG have ascending projections to the centrolateral, centromedial, dorsal hypothalamic, reuniens, Pf, paracentral, and paraventricular nuclei. Furthermore, the activity of neurons in the Pf, mediodorsal, centromedial and centrolateral nuclei can be inhibited with electrical stimulation of the PAG and somatosensory cortex. Andersen (1986) showed that inhibitory postsynaptic potentials produced by electrical stimulation of PAG override excitatory responses in the thalamus evoked by nociceptor stimulation.
Injections of HRP into the PAG (Marchand and Hagino, 1983) demonstrated the reciprocal pathway between medial thalamic nuclei and the PAG. Labeled neurons following PAG injections were in the Pf and the dorsal premamillary and dorsomedial and ventromedial nuclei of the hypothalamus. Also, electrical stimulation of Pf increases local cerebral glucose utilization in the PAG and deep layers of the superior colliculus (Aiko et al., 1987). An electrophysiological study by Sakata et al. (1988) showed that 80% of the neurons in the PAG and adjacent reticular formation produced excitatory responses following electrical stimulation of Pf and 91% of neurons that responded to peripheral noxious stimuli were also driven by Pf stimulation. Thus, there are reciprocal connections between the medial thalamic nuclei and the PAG. While the descending projection is excitatory, the ascending projection from the PAG appears to be inhibitory. It is possible that the reciprocal connections between these areas modulate responses to noxious stimuli and pain perception at cortical levels.
Medial & intralaminar thalamic projections to ACC. The earliest evidence that ACC may be directly linked to the pain system arose from studies of the monkey that showed projections of the MITN were very prominent to anterior but not posterior cingulate cortex (Vogt et al., 1979, 1987). Work with an acute rabbit preparation for analysis of responses to noxious stimuli directs the attention of this discussion to the projections of thalamic nuclei in the rabbit that likely receive spinothalamic afferents and contain nociceptive neurons. As discussed above, these include the centrolateral, Pf, paraventricular, parataenial, reuniens, submedial and ventromedial nuclei. A rabbit case with a large HRP injection mainly into rostral area 24b is presented in Figure 3. In this case there is substantial labeling of neurons in the reuniens, submedial and ventromedial nuclei and moderate labeling in the centrolateral, Pf, and parataenial nuclei. The only nucleus that has been reported to receive spinothalamic afferents in other species and does not contain labeled neurons in this case is the paraventricular nucleus. Other MITN that contain labeled neurons are the central and rhomboid nuclei.
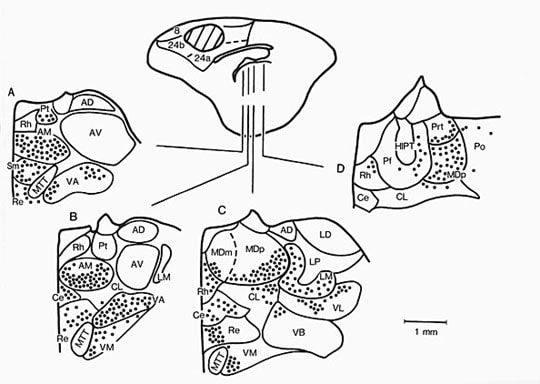
Figure 3. Horseradish peroxidase injection into rabbit area 24b and limited spread into adjacent areas 8 and 24a (hatched region). Four coronal sections through the thalamus are shown and retrogradely-labeled neurons indicated with dots (1 dot = 4 labeled neurons). Although most labeled neurons were in the anteromedial (AM), ventral anterior (VA) and parvicellular division of the mediodorsal (MDp) nuclei, many were also labeled in nuclei that receive spinothalamic afferents: parataenial (Pt), submedial (Sm), reuniens (Re), centrolateral (CL), and parafascicular (Pf) nuclei. Other abbreviations: AD, anterodorsal; AV, anteroventral; Ce, central; HIPT, habenulointerpeduncular tract; LD, laterodorsal; LM, lateral magnocellular; LP, lateral posterior; MDm, magnocellular medial dorsal; MTT, mamillothalamic tract; PrT, anterior pretectal; Po, posterior; Rh, rhomboid; VA, ventral anterior; VB, ventrobasal; VL, ventral lateral; VM, ventromedial.
Yasui et al. (1988) observed retrogradely labeled neurons ventral to the ventrobasal complex following injections in cat ACC. They suggested these neurons provide a source of nociceptive information to cingulate cortex and it is possible nociceptive information is derived from lamina I spinal cord neurons, since lamina I neurons project to the ventral part of the ventrobasal complex (Craig, 1991). Although neurons are occasionally labeled in the rabbit ventrobasal complex following injections into cingulate cortex (Vogt et al., 1992c; Fig. 1), these neurons are not located ventral to the ventrobasal complex. Furthermore, since labeled neurons have not been reported in this region of the monkey thalamus following cingulate injections (Vogt et al., 1979, 1987; Baleydier and Maguiere, 1980), it is possible that this projection is unique to the cat. Another point to consider is that neurons in the monkey Pf and limitans nuclei that do label following anterior cingulate injections of retrograde tracers may be part of a ventral system of intralaminar neurons that are phylogenetically similar to those that are ventral to the cat ventrobasal nucleus.
The proportionate contribution of inputs from nuclei that receive spinothalamic input versus those that do not has been assessed in the rabbit (Vogt et al., 1993 in Fig. 10.2). These data are an elaboration of a rabbit case that received injections of three fluorescent dyes into different rostrocaudal levels of area 24b. As shown below, it is the rostral part of area 24b that has pronounced responses to noxious stimuli, and so the projections to cortex involved by injections #1 and #2 are most interesting in the framework of the medial pain system. The input to these parts of area 24b as a percentage of all labeled neurons in the thalamus is greatest from the anteromedial, ventral anterior and parvicellular division of the mediodorsal nucleus, i.e., 15-28% of all thalamic neurons labeled. Projections from nuclei that receive spinothalamic afferents form a second tier of afferents and include the reuniens, ventromedial, centrolateral, submedial and Pf nuclei with 2-13% of the total labeled neurons. The projections of the submedial nucleus are very prominent to the rostral part of area 24b. In light of the nociceptive properties of MITN neurons and their projections to ACC, it is not surprising that neurons in area 24b have nociceptive properties and these responses depend on the medial thalamus.
The monkey ACC appears to receive proportionately more inputs from the MITN than from the AM, MD, and VA nuclei. Of the hundreds of cases we have used to study cingulate and thalamic connections in monkey, rat, and rabbit, the HRP injection into ACC shown in Figure 4 is the most important. A comparison of retrograde labeling following HRP injections into ACC and PCC shows the following nuclei project to ACC: central densocellular (Cdc), central latocellular (Clc), central lateral (Cl), reunions (Re), central inferior (Cif), central superior (Cs), paraventricular (Pv), parataenial (Pt), Pf, and limitans (Li) (Vogt et al., 1987).
Yasui et al. (1988) observed retrogradely labeled neurons ventral to the ventrobasal complex following injections in cat ACC. They suggested these neurons provide a source of nociceptive information to cingulate cortex and it is possible nociceptive information is derived from lamina I spinal cord neurons, since lamina I neurons project to the ventral part of the ventrobasal complex (Craig, 1991). Although neurons are occasionally labeled in the rabbit ventrobasal complex following injections into cingulate cortex (Vogt et al., 1992c; Fig. 1), these neurons are not located ventral to the ventrobasal complex. Furthermore, since labeled neurons have not been reported in this region of the monkey thalamus following cingulate injections (Vogt et al., 1979, 1987; Baleydier and Maguiere, 1980), it is possible that this projection is unique to the cat. Another point to consider is that neurons in the monkey Pf and limitans nuclei that do label following anterior cingulate injections of retrograde tracers may be part of a ventral system of intralaminar neurons that are phylogenetically similar to those that are ventral to the cat ventrobasal nucleus.
The proportionate contribution of inputs from nuclei that receive spinothalamic input versus those that do not has been assessed in the rabbit (Vogt et al., 1993 in Fig. 10.2). These data are an elaboration of a rabbit case that received injections of three fluorescent dyes into different rostrocaudal levels of area 24b. As shown below, it is the rostral part of area 24b that has pronounced responses to noxious stimuli, and so the projections to cortex involved by injections #1 and #2 are most interesting in the framework of the medial pain system. The input to these parts of area 24b as a percentage of all labeled neurons in the thalamus is greatest from the anteromedial, ventral anterior and parvicellular division of the mediodorsal nucleus, i.e., 15-28% of all thalamic neurons labeled. Projections from nuclei that receive spinothalamic afferents form a second tier of afferents and include the reuniens, ventromedial, centrolateral, submedial and Pf nuclei with 2-13% of the total labeled neurons. The projections of the submedial nucleus are very prominent to the rostral part of area 24b. In light of the nociceptive properties of MITN neurons and their projections to ACC, it is not surprising that neurons in area 24b have nociceptive properties and these responses depend on the medial thalamus.
The monkey ACC appears to receive proportionately more inputs from the MITN than from the AM, MD, and VA nuclei. Of the hundreds of cases we have used to study cingulate and thalamic connections in monkey, rat, and rabbit, the HRP injection into ACC shown in Figure 4 is the most important. A comparison of retrograde labeling following HRP injections into ACC and PCC shows the following nuclei project to ACC: central densocellular (Cdc), central latocellular (Clc), central lateral (Cl), reunions (Re), central inferior (Cif), central superior (Cs), paraventricular (Pv), parataenial (Pt), Pf, and limitans (Li) (Vogt et al., 1987).
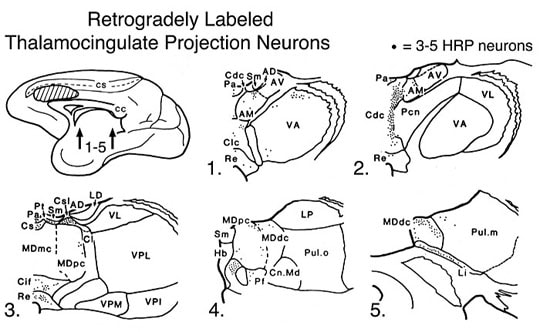
Figure 4. Distribution of retrogradely labeled neurons with HRP in the thalamus following a large injection into monkey ACC as discussed in the text. Additional abbreviations: VA, ventral anterior; VL, ventral lateral; CnMd, centrum medianum; Pul, pulvinar; Sm, stria medullaris.
It is important to reiterate that some MITN that project to ACC contain nociceptive neurons. Neurons in Pf, Cl, Li, and MD contain nociceptive neurons and a subset respond to brief taps to the skin. Thus, the MITN are likely the primary, if not only, source of nociceptive information to ACC.
It is important to reiterate that some MITN that project to ACC contain nociceptive neurons. Neurons in Pf, Cl, Li, and MD contain nociceptive neurons and a subset respond to brief taps to the skin. Thus, the MITN are likely the primary, if not only, source of nociceptive information to ACC.
Anterior Cingulate Cortex
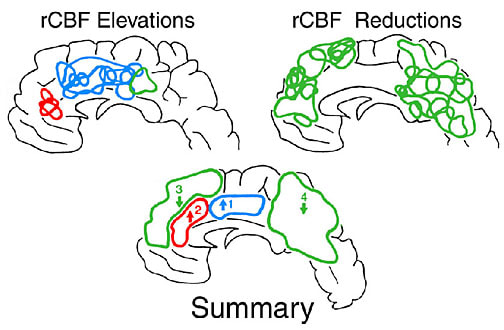
Human ACC responses to noxious and innocuous stimuli. The ACC is one of the most frequently activated cerebral cortices in studies of acute human pain processing. The most often activated part of the cingulate gyrus has been MCC (Jones et al., 1991; Talbot et al., 1991; Coghill et al., 1994; Casey et al., 1994; Silverman et al., 1997; Svensson et al., 1997). A positron emission tomography study of regional cerebral blood flow (rCBF) in individuals during noxious heat stimulation to the back of the hand elevated flow in MCC and pACC (Vogt et al., 1996). Figure 5 shows a summary of the anatomical distribution of elevations and decreases in rCBF in this study. Sites were included in the summary wherever there was an overlap of two or more sites in the 6 cases in either hemisphere. The sites of elevated rCBF in posterior cingulate cortex do not appear because there were so few and most cases had massive reductions in the posterior cingulate region. All cases had at least one MCC activation site in the right hemisphere and most had a right pACC and, in some instances, a left hemisphere pACC.
Figure 5. Distribution of increases and decreases in rCBF on the medial surface during noxious heat stimulation of the dorsal surface of the right hand. Within each major anatomical site, the summary shows two sites of statistically significant increases (1 and 2) and reductions (3 and 4) in rCBF shown in the group statistical parametric mapping analysis (Vogt et al., 1996).
The specific role of ACC in pain processing is particularly compelling in the thermal grill studies of Craig et al. (1996). Although the thermal grill employs a grid of innocuous hot and cold bars, it produces the perception of painful burning when the hand or forearm is placed across multiple bars. During this illusion, rCBF in the anterior insula is elevated as it is during stimulation with both innocuous stimuli and noxious stimuli. In contrast, rCBF in ACC is only elevated by noxious stimuli and the thermal grill illusion; not the component innocuous stimuli. Therefore, it is likely that the pain perception generated by the grill is evoked in ACC. Interestingly, the site of activation in ACC is in the dorsal part of pACC; a region that may be involved in a number of affective functions discussed in the four-region model of cingulate cortex functions.
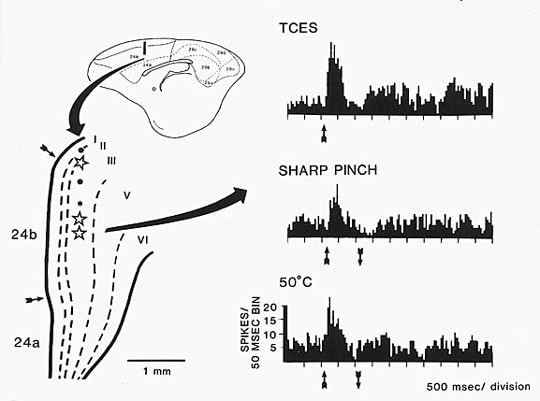
Although event-related fMRI is attaining a high degree of temporal resolution in localizing nocieptive responses, evoked potentials to noxious transcutaneous electrical stimulation provides the highest temporal resolution of these events. By subtracting the surface potentials evoked by stimulating the sural nerve or finger from potentials generated by stimulating them with a pain-threshold level of stimulation from that generated with a supra-pain the a negative difference potential is generated (Dowman and Schell, 1999). The source of this negative difference potential has been identified with dipole source localization with best fit to two sources; one located in MCC and the other to medial parietal cortex; also termed supplementary somatosensory cortex. This methodology has a bright future for non-invasive diagnosis of chronic pain syndromes; most of which impact MCC.
Noxious stimulation does not equally activate the MCC region. Davis et al. (1997) used functional MRI to show that noxious transcutaneous electrical stimulation (TCES) of the median nerve activates a posterior part of MCC and no signal is detected with innocuous electrical stimulation. When the same subjects engaged in a cognitive task like silent word generation, a more rostral part of MCC was activated. Indeed, the first study to suggest a role for cingulate cortex in divided-task processing was that of Corbetta et al. (1991) who evaluated activations during joint processing of a pair of cognitive tasks. Raichle et al. (1994) showed that verb generation to a list of nouns activated a dorsal part of ACC including the anterior part of MCC and this activity dissipated with practice. Finally, Bench et al. (1993) and Derbyshire et al. (1998) activated rostral parts of MCC and a review of the cognitive functions of rostral MCC including a thorough meta analysis is provided by Bush et al. (2000). This work suggests that posterior MCC is involved in pain processing, while the anterior division of this region is involved in cognitive processing.
In light of the many human imaging studies that activate cingulate cortex with mainly noxious stimuli and the experimental animal studies of the MITN and those of rabbit cingulate cortex detailed below, one might expect purely nociceptive responses in cingulate cortex. Over the past 5 years, however, there has been a growing body of findings that stimulus intensity coding is embedded in the ACC signal (specifically posterior MCC) and that innocuous stimuli are associated with ACC activation. To what extent is the ACC signal a nociceptive sensory signal? Becerra et al. (1999) evoked MCC activation with pulses of innocuous thermal stimuli (35° to 41° C steps), Kwan et al. (2000) activated many parts of ACC with innocuous cutaneous thermal stimuli, and Aziz et al. (2000) activated dorsal MCC with innocuous stimulation of the esophagus. Studies showing that an intensity code is part of the ACC response emphasize this view. Derbyshire et al. (1997) reported that three levels of cutaneous pain evoked with a laser stimulator (i.e., threshold, mild, and moderate) evoked a progressively higher level of blood flow in pACC, while Coghill et al. (1999) showed intensity coding for MCC. This latter study suggested that all nociceptive telencephalic regions have an intensity code. This need not mean, however, that every region is equally involved in intensity perception nor that any one region’s primary function is intensity coding. The intensity code in cingulate cortex subserves its primary functions of affect regulation and behavioral response modification. In addition, anterior MCC can be activated with innocuous stimulation, however, such activity may not be a sensory code per se but rather activity associated with cognitive processing functions of MCC.
An alternative view to the nociceptive, sensory-coding hypothesis of MCC function is the view that pACC is involved in affective and posterior MCC in motivational functions associated with autonomic and skeletomotor activity, respectively. In this context, the sensory coding properties in parts of cingulate cortex are simply an adjunct to triggering different motor outcomes depending on the behavioral relevance to each level of sensory stimulation. According to the motor hypothesis of MCC function, it is the coding of the negative or positive properties of the reward that guides particular behaviors. If a wide range of behaviors is guided by cingulate cortex, a wide the range of sensory stimuli may be needed for directing those behaviors (i.e., innocuous and noxious). Bush et al. (2001) provide evidence for a role for ACC in this function and the primary role of MCC in response selection in the four-region, structure-function model of cingulate cortex emphasizes the role of cingulate cortex in selecting among motor options and modifying behavior according to changing reward properties of particular stimuli, including noxious ones.
To the extent that sensory signals are used by cingulate cortex as a motivational signal to enhance life-sustaining responses and avoid life-threatening stimuli, activity in this region must be under cognitive control. Thus, Corbetta et al. (1991) first demonstrated that cingulate cortex activity is enhanced in tasks that have a high level of cognitive demand and Peyron et al. (1999) made an important extrapolation of this work to pain processing. The latter investigators showed that MCC responses to noxious stimuli are not cognitively stable but they can be modified by the subject when a cognitive shift is made to or from consciously rating the intensity of noxious stimuli or an auditory counting task. It is assumed that, if the nociceptive signal in MCC were strictly a sensory, intensity-coding signal, the response would not be modified by cognitive challenge as it is in the insula and second somatosensory cortex (Peyron et al., 1999), it would not undergo habituation, and receptive fields would not be so extensive.
It is concluded from human studies that nociceptive signals in pACC and posterior MCC are engaged in reorganizing autonomic and skeletomotor responses, respectively. The signal is part of a cognitive strategy that provides for changes in behavior according to changes in its reward outcomes and the pain signal is a behavioral mismatch detector. Although the ACC nociceptive signal is not primarily a sensory coding signal, a sensory code drives motivationally relevant changes in behavior.
Pain relief following cingulate lesions. The outcome of neurosurgical lesions provided the first evidence of a direct role of cingulate cortex in pain processing. This strategy developed from surgical observations that lesions of cingulate cortex and/or the cingulum bundle were useful for the treatment of psychiatric disorders such as depression, anxiety, mania and obsessive-compulsive disorders. A case of childhood obsessive-compulsive disorder associated with cingulate epilepsy was resolved with cingulotomy (Levin and Duchowny, 1991) and provocation of obsessive-compulsive symptoms elevated rCBF in ACC (Rauch et al., 1994). Postsurgical observations of psychiatric patients led to the hypothesis that such lesions also alleviate chronic, intractable pain (e.g., Lewin, 1961; Ballantine et al., 1967) and these operations are suggested as viable approaches for the treatment of pain in carefully-selected patient populations following a full range of nondestructive procedures (Gybels and Sweet, 1989).
Ballantine’s case 18 (Corkin, 1980) provides the most direct evidence that cingulotomy alleviates peripherally-generated pain. This patient had no history of illness, depression or other psychiatric disorders. He experienced persistent and disabling pain following a traumatic amputation of the left forearm that could not be relieved by peripheral nerve block or drug therapies. Immediately following cingulotomy he claimed complete relief from the stump pain and eight years later felt “great” and was working full time. Cingulotomies have been performed in a large number of patients for the treatment of pain produced by neoplastic and non-neoplastic conditions (Ballantine et al., 1967; Hurt and Ballantine, 1974; Corkin, 1980). These operations result in relief of pain in as many as 90% of nonpsychiatric patients. In some patients depression follows chronic pain and both the pain and depression are alleviated with cingulotomy. The pain relief that is attained with this procedure suggests that a noxious stimulus such as a cancer can still be perceived, however, the patient is no longer concerned with its presence, i.e., the affective response to it has been reduced.
Two variations on lesions in cingulate cortex have also been successfully employed for the relief of pain. In one, attempts were made to restrict the lesions to the cingulum bundle in what is termed a cingulumotomy. Of 12 patients with varying amounts of emotional disturbances, ten were reported to have fair to excellent relief of pain (Foltz and White, 1962, 1968). It should be noted, however, that histological demonstrations of these lesions show they vary from a “typical” lesion that involves only a part of the arcuate extension of the cingulum bundle (Foltz and White, 1962, Fig. 4) to virtually complete destruction of white matter underlying cingulate cortex including the cingulum bundle (Foltz and White, 1968, Fig. 9). Another application of cingulotomies has been to combine them with thalamotomy and other lesions including thoracic rhizotomy, and cervical cordotomy (Turnbull, 1972; Amromin et al., 1975). Though relief of intractable pain is reported in these cases, the role of ACC in the surgical response cannot be determined.
In conclusion, the verbal reports of human patients are crucial for specifying the content of pain sensations. Alterations in rCBF in pACC and MCC following noxious stimuli suggest that cingulate cortex receives nociceptor-driven afferent information. Furthermore, the alleviation of phantom limb pain and pain produced by cancers and other chronic diseases with cingulotomy directly implicates cingulate cortex in pain perception and affective responses to noxious stimuli.
Anterior cingulotomy in animals; Reductions in pain-behaviors and avoidance learning. Animal models of chronic pain suggest that manipulation of cingulate cortex and/or the cingulum bundle can alter responses to pain. Vaccarino and Melzack (1989) induced pain in rats with formalin injections into one hindpaw. Lidocaine injections into the cingulum bundle significantly reduced responses to formalin, but did not alter the latency of hindpaw “flicking” following withdrawal of the paw from 50°C water. The former test is a measure of chronic pain, while the latter is considered a measure of acute pain. Fuchs et al. (1996) confirmed involvement of the cingulum bundle in the formalin model by reducing pain behaviors with electrical stimulation of this tract. In addition, Donahue et al. (2001) showed that ablations of ACC greatly reduced behaviors associated with formalin-induced inflammatory pain, while leaving intact responses to neuropathic pain produced by ligation of the fifth lumbar nerve. In many ways, these animal studies confirm the role of ACC in pain behaviors and provide a foundation for assessing the role of ACC in avoidance behaviors; i.e., those behaviors associated with the prediction and avoidance of noxious stimuli. Donahue et al. (2001) make the important point that the effectiveness of cingulotomy lesions in the rat inflammatory model of pain may be due in part to its ability to reduce anxiety about the ongoing and unavoidable noxious stimulation.
The influence of cingulate cortex lesions on avoidance learning has been assessed in the rabbit (Gabriel et al., 1991; Gabriel, 1993). In this paradigm an animal learns to avoid noxious footshock by stepping or hopping in a wheel when it hears one of two different frequency tones. Lesions of ACC produce a profound impairment in task acquisition, while lesions in posterior cingulate cortex alter the performance of this task, but not its acquisition. Since these animals are not impaired in measures of sensory or motor function, it is presumed that ACC lesions interfere with either of the following two processes. There may be an impairment in the interpretation of the consequences of noxious stimulation as potentially tissue damaging and an avoidance response is not generated. Alternatively, pairing of the noxious stimulus with the conditional tone stimulus may be impaired so that the footshock cannot be predicted as noxious and it cannot be avoided. In either instance, there is a failure of the animal to avoid the noxious stimulus and this is related coding the outcome of a particular behavior.
Gabriel and his colleagues also assessed neuronal plasticities in cingulate cortex during the acquisition and performance of discriminative avoidance learning. The crucial point in this regard is that neurons in ACC, the mediodorsal nucleus of the thalamus, and the laterobasal nucleus of the amygdala are among the first to produce excitatory discharges that distinguish between the positive and negative conditional tone stimuli (Gabriel, 1990; Gabriel et al., 1980). Thus, neurons in area 24 produce discriminative discharges during the acquisition phase of the task. In contrast, neurons in posterior cingulate cortex distinguish between these stimuli later in training when performance of this task is the dominant behavioral activity.
Cingulate cortex has also been implicated in aversive classical conditioning. Of particular note here is the fact that neurons in dorsal and anterior parts of area 24 undergo training-induced changes in activity during aversive conditioning (Gibbs and Powell, 1991). In this study, cingulate unit discharges and heart rate parameters were monitored during training with two distinctive tones. The positive conditional stimulus was one tone paired with eyeshock and the negative conditional stimulus was another tone that was never paired with eyeshock. Changes in area 24 neuronal activity appeared to reflect the differential contingencies of the positive and negative conditional stimuli.
These lesion and recording observations taken together suggest that ACC is involved in the early acquisition of discriminations that predict noxious electrical stimulation. The avoidance behavior itself is critically dependent on neurons in area 24 and the early discharges of neurons in the amygdala and thalamus suggest that these structures may interact with ACC as a network in discriminative avoidanceprocesses. Finally, it appears that autonomic and skeletomotor responses to noxious stimuli are organized in the same part of dorsal ACC, i.e., area 24b. This is a region that contains most nociceptive neurons in the rabbit as discussed below. This is somewhat different from the human where these two functional fields appear to be segregated into the pACC and MCC.
The specific role of ACC in pain processing is particularly compelling in the thermal grill studies of Craig et al. (1996). Although the thermal grill employs a grid of innocuous hot and cold bars, it produces the perception of painful burning when the hand or forearm is placed across multiple bars. During this illusion, rCBF in the anterior insula is elevated as it is during stimulation with both innocuous stimuli and noxious stimuli. In contrast, rCBF in ACC is only elevated by noxious stimuli and the thermal grill illusion; not the component innocuous stimuli. Therefore, it is likely that the pain perception generated by the grill is evoked in ACC. Interestingly, the site of activation in ACC is in the dorsal part of pACC; a region that may be involved in a number of affective functions discussed in the four-region model of cingulate cortex functions.
Although event-related fMRI is attaining a high degree of temporal resolution in localizing nocieptive responses, evoked potentials to noxious transcutaneous electrical stimulation provides the highest temporal resolution of these events. By subtracting the surface potentials evoked by stimulating the sural nerve or finger from potentials generated by stimulating them with a pain-threshold level of stimulation from that generated with a supra-pain the a negative difference potential is generated (Dowman and Schell, 1999). The source of this negative difference potential has been identified with dipole source localization with best fit to two sources; one located in MCC and the other to medial parietal cortex; also termed supplementary somatosensory cortex. This methodology has a bright future for non-invasive diagnosis of chronic pain syndromes; most of which impact MCC.
Noxious stimulation does not equally activate the MCC region. Davis et al. (1997) used functional MRI to show that noxious transcutaneous electrical stimulation (TCES) of the median nerve activates a posterior part of MCC and no signal is detected with innocuous electrical stimulation. When the same subjects engaged in a cognitive task like silent word generation, a more rostral part of MCC was activated. Indeed, the first study to suggest a role for cingulate cortex in divided-task processing was that of Corbetta et al. (1991) who evaluated activations during joint processing of a pair of cognitive tasks. Raichle et al. (1994) showed that verb generation to a list of nouns activated a dorsal part of ACC including the anterior part of MCC and this activity dissipated with practice. Finally, Bench et al. (1993) and Derbyshire et al. (1998) activated rostral parts of MCC and a review of the cognitive functions of rostral MCC including a thorough meta analysis is provided by Bush et al. (2000). This work suggests that posterior MCC is involved in pain processing, while the anterior division of this region is involved in cognitive processing.
In light of the many human imaging studies that activate cingulate cortex with mainly noxious stimuli and the experimental animal studies of the MITN and those of rabbit cingulate cortex detailed below, one might expect purely nociceptive responses in cingulate cortex. Over the past 5 years, however, there has been a growing body of findings that stimulus intensity coding is embedded in the ACC signal (specifically posterior MCC) and that innocuous stimuli are associated with ACC activation. To what extent is the ACC signal a nociceptive sensory signal? Becerra et al. (1999) evoked MCC activation with pulses of innocuous thermal stimuli (35° to 41° C steps), Kwan et al. (2000) activated many parts of ACC with innocuous cutaneous thermal stimuli, and Aziz et al. (2000) activated dorsal MCC with innocuous stimulation of the esophagus. Studies showing that an intensity code is part of the ACC response emphasize this view. Derbyshire et al. (1997) reported that three levels of cutaneous pain evoked with a laser stimulator (i.e., threshold, mild, and moderate) evoked a progressively higher level of blood flow in pACC, while Coghill et al. (1999) showed intensity coding for MCC. This latter study suggested that all nociceptive telencephalic regions have an intensity code. This need not mean, however, that every region is equally involved in intensity perception nor that any one region’s primary function is intensity coding. The intensity code in cingulate cortex subserves its primary functions of affect regulation and behavioral response modification. In addition, anterior MCC can be activated with innocuous stimulation, however, such activity may not be a sensory code per se but rather activity associated with cognitive processing functions of MCC.
An alternative view to the nociceptive, sensory-coding hypothesis of MCC function is the view that pACC is involved in affective and posterior MCC in motivational functions associated with autonomic and skeletomotor activity, respectively. In this context, the sensory coding properties in parts of cingulate cortex are simply an adjunct to triggering different motor outcomes depending on the behavioral relevance to each level of sensory stimulation. According to the motor hypothesis of MCC function, it is the coding of the negative or positive properties of the reward that guides particular behaviors. If a wide range of behaviors is guided by cingulate cortex, a wide the range of sensory stimuli may be needed for directing those behaviors (i.e., innocuous and noxious). Bush et al. (2001) provide evidence for a role for ACC in this function and the primary role of MCC in response selection in the four-region, structure-function model of cingulate cortex emphasizes the role of cingulate cortex in selecting among motor options and modifying behavior according to changing reward properties of particular stimuli, including noxious ones.
To the extent that sensory signals are used by cingulate cortex as a motivational signal to enhance life-sustaining responses and avoid life-threatening stimuli, activity in this region must be under cognitive control. Thus, Corbetta et al. (1991) first demonstrated that cingulate cortex activity is enhanced in tasks that have a high level of cognitive demand and Peyron et al. (1999) made an important extrapolation of this work to pain processing. The latter investigators showed that MCC responses to noxious stimuli are not cognitively stable but they can be modified by the subject when a cognitive shift is made to or from consciously rating the intensity of noxious stimuli or an auditory counting task. It is assumed that, if the nociceptive signal in MCC were strictly a sensory, intensity-coding signal, the response would not be modified by cognitive challenge as it is in the insula and second somatosensory cortex (Peyron et al., 1999), it would not undergo habituation, and receptive fields would not be so extensive.
It is concluded from human studies that nociceptive signals in pACC and posterior MCC are engaged in reorganizing autonomic and skeletomotor responses, respectively. The signal is part of a cognitive strategy that provides for changes in behavior according to changes in its reward outcomes and the pain signal is a behavioral mismatch detector. Although the ACC nociceptive signal is not primarily a sensory coding signal, a sensory code drives motivationally relevant changes in behavior.
Pain relief following cingulate lesions. The outcome of neurosurgical lesions provided the first evidence of a direct role of cingulate cortex in pain processing. This strategy developed from surgical observations that lesions of cingulate cortex and/or the cingulum bundle were useful for the treatment of psychiatric disorders such as depression, anxiety, mania and obsessive-compulsive disorders. A case of childhood obsessive-compulsive disorder associated with cingulate epilepsy was resolved with cingulotomy (Levin and Duchowny, 1991) and provocation of obsessive-compulsive symptoms elevated rCBF in ACC (Rauch et al., 1994). Postsurgical observations of psychiatric patients led to the hypothesis that such lesions also alleviate chronic, intractable pain (e.g., Lewin, 1961; Ballantine et al., 1967) and these operations are suggested as viable approaches for the treatment of pain in carefully-selected patient populations following a full range of nondestructive procedures (Gybels and Sweet, 1989).
Ballantine’s case 18 (Corkin, 1980) provides the most direct evidence that cingulotomy alleviates peripherally-generated pain. This patient had no history of illness, depression or other psychiatric disorders. He experienced persistent and disabling pain following a traumatic amputation of the left forearm that could not be relieved by peripheral nerve block or drug therapies. Immediately following cingulotomy he claimed complete relief from the stump pain and eight years later felt “great” and was working full time. Cingulotomies have been performed in a large number of patients for the treatment of pain produced by neoplastic and non-neoplastic conditions (Ballantine et al., 1967; Hurt and Ballantine, 1974; Corkin, 1980). These operations result in relief of pain in as many as 90% of nonpsychiatric patients. In some patients depression follows chronic pain and both the pain and depression are alleviated with cingulotomy. The pain relief that is attained with this procedure suggests that a noxious stimulus such as a cancer can still be perceived, however, the patient is no longer concerned with its presence, i.e., the affective response to it has been reduced.
Two variations on lesions in cingulate cortex have also been successfully employed for the relief of pain. In one, attempts were made to restrict the lesions to the cingulum bundle in what is termed a cingulumotomy. Of 12 patients with varying amounts of emotional disturbances, ten were reported to have fair to excellent relief of pain (Foltz and White, 1962, 1968). It should be noted, however, that histological demonstrations of these lesions show they vary from a “typical” lesion that involves only a part of the arcuate extension of the cingulum bundle (Foltz and White, 1962, Fig. 4) to virtually complete destruction of white matter underlying cingulate cortex including the cingulum bundle (Foltz and White, 1968, Fig. 9). Another application of cingulotomies has been to combine them with thalamotomy and other lesions including thoracic rhizotomy, and cervical cordotomy (Turnbull, 1972; Amromin et al., 1975). Though relief of intractable pain is reported in these cases, the role of ACC in the surgical response cannot be determined.
In conclusion, the verbal reports of human patients are crucial for specifying the content of pain sensations. Alterations in rCBF in pACC and MCC following noxious stimuli suggest that cingulate cortex receives nociceptor-driven afferent information. Furthermore, the alleviation of phantom limb pain and pain produced by cancers and other chronic diseases with cingulotomy directly implicates cingulate cortex in pain perception and affective responses to noxious stimuli.
Anterior cingulotomy in animals; Reductions in pain-behaviors and avoidance learning. Animal models of chronic pain suggest that manipulation of cingulate cortex and/or the cingulum bundle can alter responses to pain. Vaccarino and Melzack (1989) induced pain in rats with formalin injections into one hindpaw. Lidocaine injections into the cingulum bundle significantly reduced responses to formalin, but did not alter the latency of hindpaw “flicking” following withdrawal of the paw from 50°C water. The former test is a measure of chronic pain, while the latter is considered a measure of acute pain. Fuchs et al. (1996) confirmed involvement of the cingulum bundle in the formalin model by reducing pain behaviors with electrical stimulation of this tract. In addition, Donahue et al. (2001) showed that ablations of ACC greatly reduced behaviors associated with formalin-induced inflammatory pain, while leaving intact responses to neuropathic pain produced by ligation of the fifth lumbar nerve. In many ways, these animal studies confirm the role of ACC in pain behaviors and provide a foundation for assessing the role of ACC in avoidance behaviors; i.e., those behaviors associated with the prediction and avoidance of noxious stimuli. Donahue et al. (2001) make the important point that the effectiveness of cingulotomy lesions in the rat inflammatory model of pain may be due in part to its ability to reduce anxiety about the ongoing and unavoidable noxious stimulation.
The influence of cingulate cortex lesions on avoidance learning has been assessed in the rabbit (Gabriel et al., 1991; Gabriel, 1993). In this paradigm an animal learns to avoid noxious footshock by stepping or hopping in a wheel when it hears one of two different frequency tones. Lesions of ACC produce a profound impairment in task acquisition, while lesions in posterior cingulate cortex alter the performance of this task, but not its acquisition. Since these animals are not impaired in measures of sensory or motor function, it is presumed that ACC lesions interfere with either of the following two processes. There may be an impairment in the interpretation of the consequences of noxious stimulation as potentially tissue damaging and an avoidance response is not generated. Alternatively, pairing of the noxious stimulus with the conditional tone stimulus may be impaired so that the footshock cannot be predicted as noxious and it cannot be avoided. In either instance, there is a failure of the animal to avoid the noxious stimulus and this is related coding the outcome of a particular behavior.
Gabriel and his colleagues also assessed neuronal plasticities in cingulate cortex during the acquisition and performance of discriminative avoidance learning. The crucial point in this regard is that neurons in ACC, the mediodorsal nucleus of the thalamus, and the laterobasal nucleus of the amygdala are among the first to produce excitatory discharges that distinguish between the positive and negative conditional tone stimuli (Gabriel, 1990; Gabriel et al., 1980). Thus, neurons in area 24 produce discriminative discharges during the acquisition phase of the task. In contrast, neurons in posterior cingulate cortex distinguish between these stimuli later in training when performance of this task is the dominant behavioral activity.
Cingulate cortex has also been implicated in aversive classical conditioning. Of particular note here is the fact that neurons in dorsal and anterior parts of area 24 undergo training-induced changes in activity during aversive conditioning (Gibbs and Powell, 1991). In this study, cingulate unit discharges and heart rate parameters were monitored during training with two distinctive tones. The positive conditional stimulus was one tone paired with eyeshock and the negative conditional stimulus was another tone that was never paired with eyeshock. Changes in area 24 neuronal activity appeared to reflect the differential contingencies of the positive and negative conditional stimuli.
These lesion and recording observations taken together suggest that ACC is involved in the early acquisition of discriminations that predict noxious electrical stimulation. The avoidance behavior itself is critically dependent on neurons in area 24 and the early discharges of neurons in the amygdala and thalamus suggest that these structures may interact with ACC as a network in discriminative avoidanceprocesses. Finally, it appears that autonomic and skeletomotor responses to noxious stimuli are organized in the same part of dorsal ACC, i.e., area 24b. This is a region that contains most nociceptive neurons in the rabbit as discussed below. This is somewhat different from the human where these two functional fields appear to be segregated into the pACC and MCC.
Figure 6. Density of nociceptive units on the medial surface of the rabbit brain. Three densities of units are indicated that responded to contralateral transcutaneous electrical stimulation. The highest density of nociceptive units was in the mid-rostrocaudal level of areas 24b and adjacent area 8. The corpus callosum is stippled.
Nociceptive neurons in area 24b. Neuronal activity in cingulate cortex is driven by noxious stimuli and the characteristics of their receptive fields have been reported (Sikes and Vogt, 1992). Most nociceptive neurons in rabbit are in a mid-rostrocaudal level of area 24b and a map of their distribution on the medial surface of the rabbit brain is in Figure 6. These neurons responded to noxious transcutaneous electrical stimulation (TCES) of at least 6 mA to the contralateral ear. The greatest number of responsive units was in area 24b with some extension into area 8. Of the 542 units that were tested in area 24, 150 had responses to the noxious electrical stimulation. Most of these responses (87%) were excitatory and of short duration, although the activity in some units was inhibited and some extended for a longer duration. Figure 7 shows an example of an electrode track through area 24 and responses from a number of units. Two units in this track evoked excitatory and one an inhibitory response to TCES of either the contralateral and/or ipsilateral ears.
Nociceptive neurons in area 24b. Neuronal activity in cingulate cortex is driven by noxious stimuli and the characteristics of their receptive fields have been reported (Sikes and Vogt, 1992). Most nociceptive neurons in rabbit are in a mid-rostrocaudal level of area 24b and a map of their distribution on the medial surface of the rabbit brain is in Figure 6. These neurons responded to noxious transcutaneous electrical stimulation (TCES) of at least 6 mA to the contralateral ear. The greatest number of responsive units was in area 24b with some extension into area 8. Of the 542 units that were tested in area 24, 150 had responses to the noxious electrical stimulation. Most of these responses (87%) were excitatory and of short duration, although the activity in some units was inhibited and some extended for a longer duration. Figure 7 shows an example of an electrode track through area 24 and responses from a number of units. Two units in this track evoked excitatory and one an inhibitory response to TCES of either the contralateral and/or ipsilateral ears.
Figure 7. Example of an electrode track reconstruction and unit responses for a polymodal nociceptive unit whose position was marked with an electrolytic lesion. This latter unit was located in layer III of area 24b and had brisk excitatory responses to noxious levels of 50 msec TCES, 1 sec sharp pinches and thermal stimuli over 46°C. Each histogram shows the number of action potentials in each 50 msec bin for 20 stimulus presentations. Stimulus onset and offset are indicated with the arrows except for TCES for which both are indicated with a single arrow.
In 74 units tested with TCES, response onset occurred 166±11 msec (mean±S.E.M.) after the stimulus and lasted for 519±52 msec. There are a number of explanations for the long latency to onset for these responses. If these responses originate in the medial thalamus, the long-latency response onset might be accounted for by the slow conduction velocities of ascending lamina I axons that terminate in the medial thalamus. In the cat, lamina I spinothalamic tract neurons have conduction velocities of about 4 m/sec with a mean latency to the medial thalamus of about 90 msec (Craig and Kniffki, 1985). To this must be added the activation and transmission delay to cingulate cortex, although there is additional delay not accounted for in the 166 msec average time delay for cingulate neurons.
A significant proportion of the neurons in area 24b responded to high intensity mechanical stimulation. Of 221 area 24 units tested for responses to cutaneous stimulation, 77 had excitatory and seven had inhibitory responses to noxious pinches with a serrated forceps. An example of a unit that evoked excitatory discharges to sharp pinches to the ear is shown in Figure 7. Calibrated stimuli with probes that were 1.5 mm in diameter evoked responses over 300 gm. These units did not respond to innocuous stimuli of less than 300 gm nor to light touch, brushing of the skin, or movement of the hair anywhere on the dorsal surface of the rabbit. A majority of the units tested, however, did respond to firm but non-noxious tapping of the skin of the ear with a probe. A few units responded to noxious thermal stimuli over 43°C. Of 47 units so tested, nine had excitatory and only two inhibitory responses to noxious heat stimuli. Of the units tested that responded to noxious mechanical stimuli, 25% also responded to noxious heat stimulation and the unit in Figure 7 was a polymodal unit. Thus, a significant percentage of units in ACC are polymodal nociresponsive.
The duration of responses to noxious TCES or mechanical stimuli did not accurately represent the duration of these stimuli (Vogt et al., 1993). A typical response to TCES was a short duration increase in firing that outlasted the duration of the stimulus. When noxious mechanical stimuli over 1 sec in duration were applied, the response usually adapted rapidly, returning to baseline firing levels in approximately 500 msec. In one example, an area 24 neuron responded to noxious pinches with an initial excitatory burst of action potentials and, as stimulus duration was increased from 3 to 20 sec, the period of adaptation from the early peak discharge increased in duration. Thus, the relationship between stimulus and response duration was weak and adaptation of the response prior to stimulus termination was common with long-duration stimuli. Although this may be due to the anesthetized preparation used for these studies, it may also be the case that simple stimulus/response durations are not maintained in a system that is involved primarily altering motor outputs in relation to changing patterns of reward. Habituation of nociceptive activity has also been demonstrated with fMRI in awake human subjects (Becerra et al., 1999) suggesting that the habituation of similar activity in the rabbit is not an artifact of the anesthesia.
The nocireceptive fields for cingulate neurons are quite large, usually encompassing the entire dorsal surface of the rabbit. Twelve of 15 units tested evoked responses to noxious pinches anywhere on the dorsal surface and bilateral responses were seen in nearly all cells, although significant differences in the amplitude of the response to ipsilateral versus contralateral stimulation were seen in about half of the units tested. Of 33 units tested with identical intensities of TCES to each ear, ten responded more intensely to contralateral stimulation and eight responded more to ipsilateral stimulation. The remaining units had no significant difference to stimulation of either ear. In view of these large receptive fields, there appears to be little or no coding for the location of noxious stimuli on the body surface by neurons in ACC.
The majority of neurons that respond to noxious stimuli in area 24 are in layers II (28%) and III (43%). Only 17% of responsive units are in layer V and none are in layer VI, but only four have been tested in this latter layer. Of the 17% that are responsive in layer V, most of these are in the superficial part of layer V near the border with layer III. Excitatory and inhibitory responses occur in layers II, III and V with a similar proportion of about 80% excitatory units in each layer. Thus, most nociceptive neurons in ACC are in layers II and III, while those in layer V are most frequently in the superficial part of this layer.
Medial thalamic neurons mediate nociceptor-evoked activity. Since nociceptive responses of ACC neurons are similar to those of neurons in the MITN, it is possible that responses to noxious stimuli are transmitted by nuclei in the thalamus to ACC. To test this hypothesis, reversible blocks of the activity in these nuclei were performed with injections of the anesthetic lidocaine and nociceptive neuronal activity evaluated in cingulate cortex before and after these injections. These injections virtually abolish the nociceptor-evoked activity in ACC (Sikes and Vogt, 1992). The most effective injection sites appear to be in and rostral to the Pf nucleus extending to the submedial nucleus. An example of one such experiment is presented in Figure 8. For this experiment a recording electrode was cemented to an injection cannula. Units were identified in the thalamus and area 24b that responded to noxious TCES. The location of the responsive neuron in the Pf nucleus is shown with a star in Figure 8 as is the cannula tract and the response of the cortical neuron before the lidocaine injection. Two-hundred and twenty seconds after the 1 µl lidocaine injection the response of the cortical unit to stimulation was virtually abolished, while 1360 sec later the response was recovering but had not completely recovered. Thus, it is quite likely that nociceptive neurons in area 24b derive their input from neurons in the MITN including those of the Pf nucleus.
In 74 units tested with TCES, response onset occurred 166±11 msec (mean±S.E.M.) after the stimulus and lasted for 519±52 msec. There are a number of explanations for the long latency to onset for these responses. If these responses originate in the medial thalamus, the long-latency response onset might be accounted for by the slow conduction velocities of ascending lamina I axons that terminate in the medial thalamus. In the cat, lamina I spinothalamic tract neurons have conduction velocities of about 4 m/sec with a mean latency to the medial thalamus of about 90 msec (Craig and Kniffki, 1985). To this must be added the activation and transmission delay to cingulate cortex, although there is additional delay not accounted for in the 166 msec average time delay for cingulate neurons.
A significant proportion of the neurons in area 24b responded to high intensity mechanical stimulation. Of 221 area 24 units tested for responses to cutaneous stimulation, 77 had excitatory and seven had inhibitory responses to noxious pinches with a serrated forceps. An example of a unit that evoked excitatory discharges to sharp pinches to the ear is shown in Figure 7. Calibrated stimuli with probes that were 1.5 mm in diameter evoked responses over 300 gm. These units did not respond to innocuous stimuli of less than 300 gm nor to light touch, brushing of the skin, or movement of the hair anywhere on the dorsal surface of the rabbit. A majority of the units tested, however, did respond to firm but non-noxious tapping of the skin of the ear with a probe. A few units responded to noxious thermal stimuli over 43°C. Of 47 units so tested, nine had excitatory and only two inhibitory responses to noxious heat stimuli. Of the units tested that responded to noxious mechanical stimuli, 25% also responded to noxious heat stimulation and the unit in Figure 7 was a polymodal unit. Thus, a significant percentage of units in ACC are polymodal nociresponsive.
The duration of responses to noxious TCES or mechanical stimuli did not accurately represent the duration of these stimuli (Vogt et al., 1993). A typical response to TCES was a short duration increase in firing that outlasted the duration of the stimulus. When noxious mechanical stimuli over 1 sec in duration were applied, the response usually adapted rapidly, returning to baseline firing levels in approximately 500 msec. In one example, an area 24 neuron responded to noxious pinches with an initial excitatory burst of action potentials and, as stimulus duration was increased from 3 to 20 sec, the period of adaptation from the early peak discharge increased in duration. Thus, the relationship between stimulus and response duration was weak and adaptation of the response prior to stimulus termination was common with long-duration stimuli. Although this may be due to the anesthetized preparation used for these studies, it may also be the case that simple stimulus/response durations are not maintained in a system that is involved primarily altering motor outputs in relation to changing patterns of reward. Habituation of nociceptive activity has also been demonstrated with fMRI in awake human subjects (Becerra et al., 1999) suggesting that the habituation of similar activity in the rabbit is not an artifact of the anesthesia.
The nocireceptive fields for cingulate neurons are quite large, usually encompassing the entire dorsal surface of the rabbit. Twelve of 15 units tested evoked responses to noxious pinches anywhere on the dorsal surface and bilateral responses were seen in nearly all cells, although significant differences in the amplitude of the response to ipsilateral versus contralateral stimulation were seen in about half of the units tested. Of 33 units tested with identical intensities of TCES to each ear, ten responded more intensely to contralateral stimulation and eight responded more to ipsilateral stimulation. The remaining units had no significant difference to stimulation of either ear. In view of these large receptive fields, there appears to be little or no coding for the location of noxious stimuli on the body surface by neurons in ACC.
The majority of neurons that respond to noxious stimuli in area 24 are in layers II (28%) and III (43%). Only 17% of responsive units are in layer V and none are in layer VI, but only four have been tested in this latter layer. Of the 17% that are responsive in layer V, most of these are in the superficial part of layer V near the border with layer III. Excitatory and inhibitory responses occur in layers II, III and V with a similar proportion of about 80% excitatory units in each layer. Thus, most nociceptive neurons in ACC are in layers II and III, while those in layer V are most frequently in the superficial part of this layer.
Medial thalamic neurons mediate nociceptor-evoked activity. Since nociceptive responses of ACC neurons are similar to those of neurons in the MITN, it is possible that responses to noxious stimuli are transmitted by nuclei in the thalamus to ACC. To test this hypothesis, reversible blocks of the activity in these nuclei were performed with injections of the anesthetic lidocaine and nociceptive neuronal activity evaluated in cingulate cortex before and after these injections. These injections virtually abolish the nociceptor-evoked activity in ACC (Sikes and Vogt, 1992). The most effective injection sites appear to be in and rostral to the Pf nucleus extending to the submedial nucleus. An example of one such experiment is presented in Figure 8. For this experiment a recording electrode was cemented to an injection cannula. Units were identified in the thalamus and area 24b that responded to noxious TCES. The location of the responsive neuron in the Pf nucleus is shown with a star in Figure 8 as is the cannula tract and the response of the cortical neuron before the lidocaine injection. Two-hundred and twenty seconds after the 1 µl lidocaine injection the response of the cortical unit to stimulation was virtually abolished, while 1360 sec later the response was recovering but had not completely recovered. Thus, it is quite likely that nociceptive neurons in area 24b derive their input from neurons in the MITN including those of the Pf nucleus.
Figure 8. Top panel. Activity in nociceptive neurons can be almost completely blocked with lidocaine injections into the MITN. In this example, the unit is in Pf (star) and the injection cannula just medial and dorsal thereto. The injection almost completely abolished responses to TCES (220 sec after), while the response partially returned following a subsequent 19 min delay. Bottom panel. Complete severing of the connections between medial and lateral systems (i.e., between ACC and somatosensory cortex) with a scalpel blade, shown in transverse sections B-D, failed to block ipsilateral or contralateral TCES-ear evoked neuronal discharges in area 24b. Location of the nociresponsive neuron is marked with a star in the electrode track through A. Hb, habenula; PrT, pretectum; LP, lateral posterior nucleus; LG, lateral geniculate nucleus; VB, ventrobasal nucleus; VM, ventromedial nucleus; Po, posterior nucleus; CC, corpus callosum; SpS, splenial sulcus.
To insure that the nociceptive signal in cingulate cortex was not derived from posterior or lateral cortical areas, unit responses were evaluated before and after knife-cut lesions lateral and caudal to ACC. There were no significant differences in the number of responsive neurons before or after these lesions as shown in Figure 8. The only cortex remaining intact in this preparation is the orbitofrontal cortex, however, there is no evidence in the human imaging literature that activity in any part of orbitofrontal cortex is consistently associated with cingulate cortex nociceptive activation. The undercut lesion findings provide the key argument that nociceptive activity in the anterior insula and pACC/MCC are due to independent and parallel processing.
To complete the circuit to cingulate cortex, it should be noted that the MITN project primarily to layer I. The centrolateral and ventromedial thalamic nuclei project to layer I of cingulate cortex (Herkenham, 1979; Cunningham and LeVay, 1986). In addition, electrical stimulation of the centrolateral nucleus in cat evokes field potentials in areas 5 and 7 that reverse at the border between layers II and III (Rydenhag et al., 1986). It is possible, therefore, that the main pathway by which layer III nociceptive neurons are driven in ACC is via the apical dendritic tufts of pyramidal neurons that arborize in layers I and II.
Nociceptive neurons in monkey MCC. Cingulate cortex is activated during the anticipation of noxious stimulation in monkey (Koyama et al., 1998) and human (Ploghaus et al., 1999). Nishijo et al. (1997) showed that neurons in ACC code for either visual reward, aversive, or neutral objects. Interestingly, they reported that neurons responding only to reward such as food were concentrated rostral to neurons that did not differentiate among the outcome of bar pressing. These observations leave unanswered the question of whether aversive/nocifensive movement is spatially separated from movement toward positively rewarded stimuli.
Koyama et al. (2001) evaluated neuron discharges during each phase of a task that involved discriminative responses to visual stimuli that predicted either noxious, electrical stimulation or water reward. Of 196 neurons with a task-relevant response, 36 were engaged during the prediction period and can be viewed as having an anticipatory response, although not pain-specific. Seventy-one units were active during the red cue that predicted electrical stimulation and 37 were active during the pain response/avoidance. The positions of many of the responsive neurons were marked with lesions. Although most units appeared to by in MCC, some may have been in caudal parts of pACC. Most units in both regions appeared to be in the dorsal cingulate gyrus and in the cingulate sulcus, although it is not clear whether this reflects a sampling bias. It appears that, intermingled among these nocifensive units, were a separate group of neurons coding for the water reward task (visual cue, reward response, pre-reward, reward ingestion periods; 95 of the 196 units with a task-relevant response). The intermixing of neurons coded for both positive and negative rewards and associated with different behavioral consequences suggests a complex intermingling of at least two distinct networks. This suggests there is not a segregated cortical region for nociceptive/nosifensive processing as often assumed in studies of pain processing. This is a surprising observation because the mechanisms of reward and behavioral output are quite different for the two behaviors; i.e., withdrawing from a noxious stimulus versus approaching and ingesting a water reward. Intermixing of neurons with different reward properties and motor outputs emphasizes the role ofACC in selecting among motor outputs rather than simply driving motor events.
Cingulothalamic and periaqueductal gray interactions. Electrical stimulation of the medial thalamic nuclei alters responses to noxious stimuli in experimental animals (Kupers et al., 1988) and in human patients (Richardson and Akil, 1977a,b; Sano, 1977) and these nuclei project to the PAG as discussed above. Therefore, projections of ACC to this part of the thalamus may be important to modulating responses to noxious stimuli. Royce (1983a) reported that neurons in layers V and VI of ACC project to the Pf nucleus in the cat. Furthermore, many of these neurons may have a dual projection to the Pf and caudate nucleus (Royce, 1983b). Since there are reciprocal connections between some of the MITN and the PAG and the PAG modulates responses to noxious stimuli, the direct projections of ACC to the PAG and the medial thalamic nuclei are of particular importance. It is well documented that large layer V pyramidal neurons in areas 25, 24, and 32 project to the dorsal and dorsolateral divisions of the PAG in the monkey (Müller-Preuss and Jürgens, 1976; Powell, 1978; Hardy and Leichnetz, 1981a; Mantyh, 1982; An et al., 1998) and rat (Hardy and Leichnetz, 1981b; Wyss and Sripanidkulchai, 1984). Since this projection includes efferents from area 24b, nociceptive neurons in rostral area 24b are in a subregion of cingulate cortex that projects to the PAG. In addition, the projection of subgenual area 25 to the PAG is also to the dorsolateral division of the PAG but it has an additional projection to the medial and ventrolateral divisions in rat (Wyss and Sripanidkulchai, 1984; Room et al., 1985). Rostral parts of the dorsal and dorsolateral divisions of the PAG project to the nucleus reticularis magnocellularis and the ventrolateral division of the PAG projects to the nucleus raphe magnus (Beitz et al., 1983). Thus, it is possible that activation of cinguloperiaqueductal gray connection can engage PAG neurons that project to serotonergic neurons in the midbrain and so modulate responses to noxious stimuli in the spinal cord.
The projections of cingulate cortex to the PAG could serve in the conscious control of reflex responses to painful stimuli. Electrical stimulation of medial prefrontal areas in the rat, including anterior cingulate area 24b, significantly elevates response latencies in rats in the hot-plate and tail-flick tests (Hardy, 1985). Furthermore, electrical stimulation of this medial prefrontal region inhibits the activity in 78% of nociceptive PAG neurons (Hardy and Haigler, 1985). In instances where neurons were excited by noxious stimuli, electrical stimulation of the prefrontal area abolished responses to peripheral noxious stimulation.
Cholinergic Connections and Cingulate Nociception. Cholinergic connections are an important part of pain processing in the medial pain system in general and ACC in particular because of the massive cholinergic and nociceptive projections from the parabrachial nucleus to the midline and interalaminar thalamic nuclei. A large number of neurons in the parabrachial nucleus are cholinergic (Kimura et al., 1981). This nucleus receives input from fusiform-shaped, C and/or A*-fiber-driven neurons in lamina I of the spinal cord (Hylden et al., 1986) and the dorsolateral division of the PAG (Redgrave et al., 1988). In addition, neurons in the parabrachial nucleus project to the centrolateral, paracentral, paraventricular, Pf and reuniens nuclei (Saper and Loewy, 1980). Thus, cholinergic parabrachial neurons likely receive direct nociceptor-driven, lamina I spinal cord afferents and project in turn to MITN. The peripedunculopontine tegmental and cuneiform nuclei of the reticular formation also contain cholinergic neurons that project to the centrolateral and Pf nuclei of the thalamus (Hoover and Jacobowitz, 1979; Isaacson and Tanaka, 1986).
There is no information available about cholinergic modulation of MITN neuron activity, however, it is known that acetylcholine increases the discharge rate of thalamocortical projection neurons in the lateral geniculate nucleus (Kayama et al., 1986) and acetylcholine depresses the activity of interneurons in the same nucleus (McCormick and Pape, 1988). Furthermore, the receptive fields of neurons in the ventrobasal nucleus of the cat can be altered by acetylcholine and atropine (Angus-Leppan et al., 1990). Thus, units that have either wide dynamic range or nociceptive-specific responses to stimulation of the face or tooth pulp have enlarged receptive field sizes in the presence of acetylcholine and reduced receptive field sizes in the presence of atropine. In addition, the modality of receptive field activity may also change in the presence of acetylcholine, since it increases responses of neurons to noxious stimuli that previously were driven by innocuous stimuli. Thus, acetylcholine may enhance MITN neuron excitability, lower the thresholds for noxious stimuli, and initiate learning processes associated with avoidance behaviors.
Involvement of the cholinergic system in avoidance learning has been suggested by behavioral training and post hoc analyses of ligand binding. Binding of hemicholinium-3 to the high affinity choline uptake site decreases in cholinergic neurons in rats during active avoidance learning (Wenk et al., 1984). Furthermore, during the 4-5 day course of discriminative avoidance learning in rabbits, there is an increase in binding of tritiated oxotremorine-M in the presence of unlabeled pirenzepine in the anterior thalamus and mid-rostrocaudal levels of cingulate cortex (Vogt et al., 1991). This may indicate an increase in M2 binding during the course of avoidance conditioning. Thus, the cholinergic system expresses long-term plasticities in the cerebral cortex that are associated with the acquisition of active avoidance tasks, although these changes have not yet been assessed in the nociceptive region of area 24b.
To insure that the nociceptive signal in cingulate cortex was not derived from posterior or lateral cortical areas, unit responses were evaluated before and after knife-cut lesions lateral and caudal to ACC. There were no significant differences in the number of responsive neurons before or after these lesions as shown in Figure 8. The only cortex remaining intact in this preparation is the orbitofrontal cortex, however, there is no evidence in the human imaging literature that activity in any part of orbitofrontal cortex is consistently associated with cingulate cortex nociceptive activation. The undercut lesion findings provide the key argument that nociceptive activity in the anterior insula and pACC/MCC are due to independent and parallel processing.
To complete the circuit to cingulate cortex, it should be noted that the MITN project primarily to layer I. The centrolateral and ventromedial thalamic nuclei project to layer I of cingulate cortex (Herkenham, 1979; Cunningham and LeVay, 1986). In addition, electrical stimulation of the centrolateral nucleus in cat evokes field potentials in areas 5 and 7 that reverse at the border between layers II and III (Rydenhag et al., 1986). It is possible, therefore, that the main pathway by which layer III nociceptive neurons are driven in ACC is via the apical dendritic tufts of pyramidal neurons that arborize in layers I and II.
Nociceptive neurons in monkey MCC. Cingulate cortex is activated during the anticipation of noxious stimulation in monkey (Koyama et al., 1998) and human (Ploghaus et al., 1999). Nishijo et al. (1997) showed that neurons in ACC code for either visual reward, aversive, or neutral objects. Interestingly, they reported that neurons responding only to reward such as food were concentrated rostral to neurons that did not differentiate among the outcome of bar pressing. These observations leave unanswered the question of whether aversive/nocifensive movement is spatially separated from movement toward positively rewarded stimuli.
Koyama et al. (2001) evaluated neuron discharges during each phase of a task that involved discriminative responses to visual stimuli that predicted either noxious, electrical stimulation or water reward. Of 196 neurons with a task-relevant response, 36 were engaged during the prediction period and can be viewed as having an anticipatory response, although not pain-specific. Seventy-one units were active during the red cue that predicted electrical stimulation and 37 were active during the pain response/avoidance. The positions of many of the responsive neurons were marked with lesions. Although most units appeared to by in MCC, some may have been in caudal parts of pACC. Most units in both regions appeared to be in the dorsal cingulate gyrus and in the cingulate sulcus, although it is not clear whether this reflects a sampling bias. It appears that, intermingled among these nocifensive units, were a separate group of neurons coding for the water reward task (visual cue, reward response, pre-reward, reward ingestion periods; 95 of the 196 units with a task-relevant response). The intermixing of neurons coded for both positive and negative rewards and associated with different behavioral consequences suggests a complex intermingling of at least two distinct networks. This suggests there is not a segregated cortical region for nociceptive/nosifensive processing as often assumed in studies of pain processing. This is a surprising observation because the mechanisms of reward and behavioral output are quite different for the two behaviors; i.e., withdrawing from a noxious stimulus versus approaching and ingesting a water reward. Intermixing of neurons with different reward properties and motor outputs emphasizes the role ofACC in selecting among motor outputs rather than simply driving motor events.
Cingulothalamic and periaqueductal gray interactions. Electrical stimulation of the medial thalamic nuclei alters responses to noxious stimuli in experimental animals (Kupers et al., 1988) and in human patients (Richardson and Akil, 1977a,b; Sano, 1977) and these nuclei project to the PAG as discussed above. Therefore, projections of ACC to this part of the thalamus may be important to modulating responses to noxious stimuli. Royce (1983a) reported that neurons in layers V and VI of ACC project to the Pf nucleus in the cat. Furthermore, many of these neurons may have a dual projection to the Pf and caudate nucleus (Royce, 1983b). Since there are reciprocal connections between some of the MITN and the PAG and the PAG modulates responses to noxious stimuli, the direct projections of ACC to the PAG and the medial thalamic nuclei are of particular importance. It is well documented that large layer V pyramidal neurons in areas 25, 24, and 32 project to the dorsal and dorsolateral divisions of the PAG in the monkey (Müller-Preuss and Jürgens, 1976; Powell, 1978; Hardy and Leichnetz, 1981a; Mantyh, 1982; An et al., 1998) and rat (Hardy and Leichnetz, 1981b; Wyss and Sripanidkulchai, 1984). Since this projection includes efferents from area 24b, nociceptive neurons in rostral area 24b are in a subregion of cingulate cortex that projects to the PAG. In addition, the projection of subgenual area 25 to the PAG is also to the dorsolateral division of the PAG but it has an additional projection to the medial and ventrolateral divisions in rat (Wyss and Sripanidkulchai, 1984; Room et al., 1985). Rostral parts of the dorsal and dorsolateral divisions of the PAG project to the nucleus reticularis magnocellularis and the ventrolateral division of the PAG projects to the nucleus raphe magnus (Beitz et al., 1983). Thus, it is possible that activation of cinguloperiaqueductal gray connection can engage PAG neurons that project to serotonergic neurons in the midbrain and so modulate responses to noxious stimuli in the spinal cord.
The projections of cingulate cortex to the PAG could serve in the conscious control of reflex responses to painful stimuli. Electrical stimulation of medial prefrontal areas in the rat, including anterior cingulate area 24b, significantly elevates response latencies in rats in the hot-plate and tail-flick tests (Hardy, 1985). Furthermore, electrical stimulation of this medial prefrontal region inhibits the activity in 78% of nociceptive PAG neurons (Hardy and Haigler, 1985). In instances where neurons were excited by noxious stimuli, electrical stimulation of the prefrontal area abolished responses to peripheral noxious stimulation.
Cholinergic Connections and Cingulate Nociception. Cholinergic connections are an important part of pain processing in the medial pain system in general and ACC in particular because of the massive cholinergic and nociceptive projections from the parabrachial nucleus to the midline and interalaminar thalamic nuclei. A large number of neurons in the parabrachial nucleus are cholinergic (Kimura et al., 1981). This nucleus receives input from fusiform-shaped, C and/or A*-fiber-driven neurons in lamina I of the spinal cord (Hylden et al., 1986) and the dorsolateral division of the PAG (Redgrave et al., 1988). In addition, neurons in the parabrachial nucleus project to the centrolateral, paracentral, paraventricular, Pf and reuniens nuclei (Saper and Loewy, 1980). Thus, cholinergic parabrachial neurons likely receive direct nociceptor-driven, lamina I spinal cord afferents and project in turn to MITN. The peripedunculopontine tegmental and cuneiform nuclei of the reticular formation also contain cholinergic neurons that project to the centrolateral and Pf nuclei of the thalamus (Hoover and Jacobowitz, 1979; Isaacson and Tanaka, 1986).
There is no information available about cholinergic modulation of MITN neuron activity, however, it is known that acetylcholine increases the discharge rate of thalamocortical projection neurons in the lateral geniculate nucleus (Kayama et al., 1986) and acetylcholine depresses the activity of interneurons in the same nucleus (McCormick and Pape, 1988). Furthermore, the receptive fields of neurons in the ventrobasal nucleus of the cat can be altered by acetylcholine and atropine (Angus-Leppan et al., 1990). Thus, units that have either wide dynamic range or nociceptive-specific responses to stimulation of the face or tooth pulp have enlarged receptive field sizes in the presence of acetylcholine and reduced receptive field sizes in the presence of atropine. In addition, the modality of receptive field activity may also change in the presence of acetylcholine, since it increases responses of neurons to noxious stimuli that previously were driven by innocuous stimuli. Thus, acetylcholine may enhance MITN neuron excitability, lower the thresholds for noxious stimuli, and initiate learning processes associated with avoidance behaviors.
Involvement of the cholinergic system in avoidance learning has been suggested by behavioral training and post hoc analyses of ligand binding. Binding of hemicholinium-3 to the high affinity choline uptake site decreases in cholinergic neurons in rats during active avoidance learning (Wenk et al., 1984). Furthermore, during the 4-5 day course of discriminative avoidance learning in rabbits, there is an increase in binding of tritiated oxotremorine-M in the presence of unlabeled pirenzepine in the anterior thalamus and mid-rostrocaudal levels of cingulate cortex (Vogt et al., 1991). This may indicate an increase in M2 binding during the course of avoidance conditioning. Thus, the cholinergic system expresses long-term plasticities in the cerebral cortex that are associated with the acquisition of active avoidance tasks, although these changes have not yet been assessed in the nociceptive region of area 24b.
Conclusions
The history of dissociating the pain system into sensory/discriminative and affective/motivational components now finds support with functional and structural studies of medial and intralaminar thalamic nuclei and their projections to ACC. This cortical region and parts of the limbic thalamus have been conceptualized as forming the medial pain system. The medial pain structures have been directly implicated in affective responses to noxious stimuli by functional imaging and neurosurgical observations as well as a wealth of experimental animal studies showing that they are involved in the prediction and avoidance of noxious stimuli.
The demonstration of a region in ACC with a high density of nociceptive neurons validates the notion that cingulate cortex is directly linked with pain perception.
When considering the role of cingulate cortex in pain processing, it is instructive to keep in mind the four-region model. Since the human imaging data do not support a simple role in sensory processing including intensity coding, although an intensity code has been identified in this region, we must consider other contributions of cingulate cortex to pain processing. Indeed, this statement itself must be reversed. The goal is to understand how the pain signal is used by cingulate cortex to regulate autonomic and skeletomotor functions. The pain signal is a somatic notification that prediction of a particular outcome failed and there was a mismatch with expectations. Such a mismatch must generate an immediate assessment of the environment for cues that predict the painful outcome in the future and guide new avoidance behaviors. This is compatible with the conclusion by Bush et al. (2001, 2002) that cingulate cortex responds during changing reward conditions. Thus, the medial pain system concept is likely an oversimplification to the extent that it focuses on CNS organization based on sensory pain processing rather than the organism’s use of pain signals as an alert to behavioral response mismatches and the need to engage in new behaviors for changing patterns of reward.
In retrospect, it appears obvious that one or more limbic cortices would be involved in affective responses to noxious stimuli. It certainly is a surprise that the pain signal is employed in limbic cortex as a potential code for modulating plasticities in motor function. Cingulate cortex is an ideal candidate for modulating long-term changes in skeletomotor output because it has nociceptive neurons, direct cingulospinal projections, connections with key limbic structures including the cholinergic system and amygdala that are involved in learning and memory, and it can specifically be engaged for the prediction and avoidance of noxious stimuli.
The demonstration of a region in ACC with a high density of nociceptive neurons validates the notion that cingulate cortex is directly linked with pain perception.
When considering the role of cingulate cortex in pain processing, it is instructive to keep in mind the four-region model. Since the human imaging data do not support a simple role in sensory processing including intensity coding, although an intensity code has been identified in this region, we must consider other contributions of cingulate cortex to pain processing. Indeed, this statement itself must be reversed. The goal is to understand how the pain signal is used by cingulate cortex to regulate autonomic and skeletomotor functions. The pain signal is a somatic notification that prediction of a particular outcome failed and there was a mismatch with expectations. Such a mismatch must generate an immediate assessment of the environment for cues that predict the painful outcome in the future and guide new avoidance behaviors. This is compatible with the conclusion by Bush et al. (2001, 2002) that cingulate cortex responds during changing reward conditions. Thus, the medial pain system concept is likely an oversimplification to the extent that it focuses on CNS organization based on sensory pain processing rather than the organism’s use of pain signals as an alert to behavioral response mismatches and the need to engage in new behaviors for changing patterns of reward.
In retrospect, it appears obvious that one or more limbic cortices would be involved in affective responses to noxious stimuli. It certainly is a surprise that the pain signal is employed in limbic cortex as a potential code for modulating plasticities in motor function. Cingulate cortex is an ideal candidate for modulating long-term changes in skeletomotor output because it has nociceptive neurons, direct cingulospinal projections, connections with key limbic structures including the cholinergic system and amygdala that are involved in learning and memory, and it can specifically be engaged for the prediction and avoidance of noxious stimuli.
References
Aiko Y, Shima F, Hosokawa S, Kato M, Kitamura K (1987) Altered local cerebral glucose utilization induced by electrical stimulation of the thalamic sensory and parafascicular nuclei in rats. Brain Res 408:47-56
An X, Bandler R, Ongur D, Price JL (1998) Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in Macaque monkeys. J Comp Neurol 401:455-479
Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ III, Willis WD Jr. (1985) Diencephalic mechanisms of pain sensation. Brain Res Rev 9:217-296
Amromin GD, Crue BL Jr, Felsoory A, Todd EM (1975) Bilateral stereotaxic cingulotomy following thoracic rhizotomy, cervical cordotomy, and thalamotomy in a patient with intractable pain: A clinical-pathological study. In: Pain: Research and Treatment, Crue BL, ed. New York: Academic Press
Andersen E (1986) Periaqueductal gray and cerebral cortex modulate responses of medial thalamic neurons to noxious stimulation. Brain Res 375:30-36
Angus-Leppan H, Olausson B, Lambert GA (1990) Modulation of receptive fields of cat nociceptive thalamic neurons by cholinergic mechanisms. Neurosci Abs 16:566
Apkarian AV, Hodge CJ (1989) Primate spinothalamic pathways: I. A quantitative study of the cells of origin of the spinothalamic pathway. J Comp Neurol 288:447-473
Applebaum AE, Beall JE, Foreman RD, Willis WS (1975) Organization and receptive fields of primate spinothalamic tract neurons. J Neurophysiol 38:572-586
Aziz Q, Thompson DG, Ng VWK, Hamdy S, Sarkar S, Brammer MJ, Bullmore ET,, Hobson A, Tracey I Gregory L, Simmons A, Williams SCR (2000) Cortical processing of human somatic and visceral sensation. J Neurosci 20:2657-2663
Ballantine HT, Cassidy WL, Flanagan NB, Marino R Jr (1967) Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 26:488-495
Baleydier C, Mauguiere F (1980) The duality of the cingulate gyrus in monkey: Neuroanatomical study and functional hypothesis. Brain 103:525-554
Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D (1999) Human brain activation under controlled thermal stimulation and habituation to noxious heat: an MRI study. Magn Reson Med 41:1044-1057
Beitz AJ, Mullett MA, Weiner LL (1983) The periaqueductal gray projections to the rat spinal trigeminal, raphe magnus, gigantocellular pars alpha and paragigantocellular nuclei arise from separate neurons. Brain Res 288:307-314
Bench CJ, Frith CD, Grasby M, Friston KJ, Paulesu E, Frackowiack RSJ, Dolan RJ (1993) Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 31:907-922
Boivie J (1979) An anatomical reinvestigation of the termination of the spinothalamic tract in the monkey. J Comp Neurol 186:343-370
Bullitt E (1990) Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 296:517-530
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cog Sci 4:215-222
Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA (2002) The oddball Stroop: A multidimensional cognitive interference task validated with fMRI in individual subjects. Mol Psychiatr, submitted
Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2001) Dorsal anterior cingulate cortex: A role in reward-based decision-making. Proc Natl Acad Sci USA, in press
Bushnell MC, Duncan GH (1989) Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res 78:415-418
Carstens E, Hartung M, Stelzer B, Zimmerman M (1990) Suppression of a hind limb flexion withdrawal reflex by microinjection of glutamate or morphine into the periaqueductal gray in the rat. Pain 43:105-112
Carstens E, Trevino DL (1978) Anatomical and physiological properties of ipsilaterally projecting spinothalamic neurons in the second cervical segment of the cat's spinal cord. J Comp Neurol 182:167-184
Casey KL (1966) Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol 29:727-750
Casey KL (1999) Forebrain mechanisms of nociception and pain: Analysis through imaging. Proc Natl Acad Sci USA 96:7668-7674
Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA (1994) Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 71:802-807
Chudler EH, Anton F, Dubner R, Kenshalo DR Jr (1990) Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: effect of interstimulus interval. J Neurophysiol 63:559-569
Chudler EH, Dong WK, Kawakami Y (1986) Cortical nociceptive responses and behavioral correlates in the monkey. Brain Res 397:47-60
Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH (1994) Distributed processing of pain and vibration by the human brain. J Neurosci 14:4095-4108
Coghill RC, Sang CN, Maisog JM, Ladarola MJ (1999) Pain intensity processing within the human brain. J Neurophysiol 82:1934-1943
Comans PE, Snow PJ (1981) Ascending projections to nucleus parafascicularis of the cat. Brain Res 230:337-341
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Peterson SE (1991) Selective and divided attention during visual discrimination of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11:2383-2402
Corkin S (1980) A prospective study of cingulotomy. In: The Psychosurgery Debate, Valenstein ES, ed. Chap 9, San Francisco: W.H. Freeman and Co.
Craig AD Jr (1990) Nociceptive neurons in the nucleus submedius (Sm) in the medial thalamus of the cat. Pain 40 (Supp 5):S492
Craig AD Jr (1991) Supraspinal pathways and mechanisms relevant to central pain. In: Pain and Central Nervous System Disease: The Central Pain Syndromes, KL Casey, ed. Chap 12, New York: Raven Press, Ltd.
Craig AD Jr (1994) Spinal and supraspinal processing of specific pain and temperature. In: Progress in Pain Research and Management, Boivie J, Hansson P, Lindblom U, eds, IASP Press, 421-437
Craig AD Jr (1995) Supraspinal projections of lamina I neurons. In: Forebrain Areas Involved in Pain Processing. Besson JM, Guilbaud G, Ollat H, eds, John Libbey Eurotext, 13-25.
Craig AD Jr, Burton H (1981) Spinal and medullary lamina I projection to nucleus submedius in medial thalamus: a possible pain center. J Neurophysiol 45:443-466
Craig AD Jr, Hunsley SJ (1991) Morphine enhances the activity of thermoreceptive cold-specific lamina I spinothalamic neurons in the cat. Brain Res 558:93-97
Craig AD Jr., Heppelmann B, Schaible H.-G. (1988) The projection of the medial and posterior articular nerves of the cat’s knee to the spinal cord. J Comp Neurol 276:279-288
Craig AD Jr, Kniffki K-D (1985) Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol (Lond) 365:197-221
Craig AD Jr, Linington AJ, Kniffki K-D (1989) Cells of origin of spinothalamic tract projections to the medial and lateral thalamus in the cat. J Comp Neurol 289:568-585
Cunningham ET, LeVay S (1986) Laminar and synaptic organization of the projection from the thalamic nucleus centralis to primary visual cortex in the cat. J Comp Neurol 254:65-77
Dado RJ, Giesler GJ Jr (1990) Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci 10:2672-2686
Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ (1997) Functional MRI of Pain- and attention-related activations in the human cingulate cortex. J Neurophysiol 77:3370-3380
Derbyshire SWG (2000) Exploring the pain “neuromatrix.” Curr Rev Pain 4:467-477
Derbyshire SWG, Jones AKP, Gyulani F, Clark S, Townsend D, Firestone LL (1997) Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73:431-445
Derbyshire SWG, Vogt BA, Jones AKP (1998) Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118:52-60
DeYoe EA, Van Essen DC (1988) Concurrent processing streams in monkey visual cortex. Trends Neurosci 11:219-226
Donahue RR, LaGraize SC, Fuchs PN (2001) Electrolytic lesion of the anterior cingulate cortex decreases inflammatory but not neuropathic nociceptive behavior in rats. Brain Res 897:131-138
Dong WK, Ryu H, Wagman IH (1978) Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol 41:1592-1613
Dong WK, Salonen LD, Kawakami Y, Shlwaku T, Kaukoranta M, Martin RF (1989) Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res 484:314-324
Dowman R, Schell S (1999) Evidence that the anterior cingulate and supplementary somatosensory cortices generate the pain-related negative difference potential. Clin Neurophysiol 110:2117-2126.
Foltz EL, White LE (1962) Pain “relief” by frontal cingulumotomy. J Neurosurg 19:89-100
Foltz EL, White LE (1968) The role of rostral cingulumotomy in “pain” relief. Int J Neurol 6:353-373
Fuchs PN, Balinsky M, Melzack R (1996) Electrical stimulation of the cingulum bundle and surrounding cortical tissue reduces formalin-test pain in the rat. Brain Res 743:116-123
Gabriel M (1990) Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. In: Progress in Brain Research, Uylings HBM, Van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, eds. New York: Academic Press 85:467-483
Gabriel M (1993) Discriminative avoidance learning: A model system. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, Vogt BA, Gabriel M, eds, Birkhauser Boston, 478-523
Gabriel M, Foster K, Orona E, Saltwick SE, Stanton M (1980) Neuronal activity of cingulate cortex, anteroventral thalamus, and hippocampal formation in discriminative conditioning: Encoding and extraction of the significance of conditional stimuli. Prog Psychobiol Physiol Psychol 9:125-231
Gabriel M, Vogt BA, Kubota Y, Poremba A, Kang E (1991) Training-stage related neuronal plasticity in limbic thalamus and cingulate cortex during learning: A possible key to mnemonic retrieval. Behav Brain Res, in press
Gibbs CM, Powell DA (1991) Single-unit activity in the dorsomedial prefrontal cortex during the expression of discriminative bradycardia in rabbits. Behav Brain Res 43:79-92
Giesler GJ Jr, Yezierski RP, Gerhart KD, Willis WD (1981) Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: Evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol 46:1285-1308.
Gybels JM, Sweet WH (1989) Neurological treatment of persistent pain. Basel: Karger
Hardy JD (1953) Thresholds of pain and reflex contraction as related to noxious stimulation. J Appl Physiol 5:725-739
Hardy SGP (1985) Analgesia elicited by prefrontal stimulation. Brain Res 339:281-284
Hardy SGP, Haigler HJ (1985) Prefrontal influences upon the midbrain: A possible route for pain modulation. Brain Res 339:285-293
Hardy SGP, Leichnetz GR (1981a) Cortical projections to the periaqueductal gray in the monkey: A retrograde and orthograde horseradish peroxidase study. Neurosci Lett 22:97-101
Hardy SGP, Leichnetz GR (1981b) Frontal cortical projections to the periaqueductal gray in the rat: A retrograde and orthograde horseradish peroxidase study. Neurosci Lett 23:13-17
Harmann PA, Carlton SM, Willis WD (1988) Collaterals of spinothalamic tract cells to the periaqueductal gray: a fluorescent double-labeling study in the rat. Brain Res 441:87-97
Hayashi H (1985) Morphology of terminations of small and large myelinated trigeminal primary afferent fibers in the cat. J Comp Neurol 240:71-89
Herkenham M (1979) The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol 183:487-518
Hitchcock ER, Teixeira MJ (1980) A comparison of results from center-median and basal thalamotomies for pain. Surg Neurol 15:341-351
Hoover DB, Jacobowitz DM (1979) Neurochemical and histochemical studies of the effect of a lesion of the nucleus cuneiformis on the cholinergic innervation of discrete areas of the rat brain. Brain Res 170:113-122
Hurt RW, Ballantine HT Jr (1974) Stereotactic anterior cingulate lesions for persistent pain: a report on 68 cases. In: Clinical Neurosurgery, Wilkins RH, ed. Baltimore: Williams and Wilkins
Hylden JLK, Hayashi H, Dubner R, Bennett GJ (1986) Physiology and morphology of the lamina I spinomesencephalic projection. J Comp Neurol 247:505-515
Isaacson LG, Tanaka D Jr (1986) Cholinergic and non-cholinergic projections from the canine pontomesencephalic tegmentum (Ch5 area) to the caudal intralaminar thalamic nuclei. Exp Brain Res 62:179-188
Ishijima B, Yoshimasu N, Fukushima T, Hori T, Sekino H, Sano K (1975) Nociceptive neurons in the human thalamus. Confin Neurol 37:99-106
Jones AKP, Brown WD, Friston KJ, Qi LY, Frackowiak RSJ (1991) Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc R Soc Lond B 244:39-44
Kaelber WW, Mitchell CL, Yarmat AJ, Afifi AK, Lorens SA (1975) Centrum medianum-parafascicularis lesions and reactivity to noxious and non-noxious stimuli. Exper Brain Res 46:282-290
Kayama Y, Takagi M, Ogawa T (1986) Cholinergic influence of the laterodorsal tegmental nucleus on neuronal activity in the rat lateral geniculate nucleus. J Neurophysiol 56:1297-1309
Kenshalo DR Jr, Chudler EH, Anton F, Dubner R (1988) SI nociceptive neurons participate in the encoding process by whih monkeys perceive the intensity of noxious thermal stimuli. Brain Res 454:378-382
Kenshalo DR Jr, Giesler GJ Jr, Leonard RB, Willis WD (1980) Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol 43:1594-1614
Kenshalo DR, Isensee O (1983) Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol 50:1479-1496
Kenshalo DR Jr, Thomas DA, Dubner R (1991) Primary somatosensory cortical lesions reduce the monkeys' ability to discriminate and detect noxious thermal stimulation. Neurosci Abs 17:1206
Kenshalo DR, Willis WD Jr (1991) The role of the cerebral cortex in pain sensation. In: Cerebral Cortex, Peters A, Jones EG, eds. New York: Plenum Press 9:153-212
Kimura H, McGeer PL, Peng JH, McGeer EG (1981) The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol 200:151-201
Koyama T, Kato K, Tanaka YZ, Mikami A (2001) Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neurosci Res 39:412-430
Koyama T, Tanaka YZ, Mikami A (1998) Nociceptive neurons in the macaque anterior cingulate cortex during anticipation of pain. NeuroReport 9:2663-2667
Kumazawa T, Perl ER (1978) Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol 177:417-434
Kupers RC, Vos BPJ, Gybels JM (1988) Stimulation of the nucleus paraventricularis thalami suppresses scratching and biting behavior of arthritic rats and exerts a powerful effect on tests for acute pain. Pain 32:115-125
Kwan CL, Crawley AP, Mikulis DJ, Davis KD (2000) An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85:359-374
La Bars D, Dickenson AH, Besson JM (1979) Diffuse noxious inhibitory controls (DNIC) I. Effects on dorsal horn convergent neurones in the rat. II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6:283-327
Levin B, Duchowny M (1991) Childhood obsessive-compulsive disorder and cingulate epilepsy. Biol Psychiatr 30:1049-1055
Lewin W (1961) Observations on selective leukotomy. J Neurol Neurosurg Psychiat 24:37-44
Light AR, Perl ER (1979) Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186:133-150
Liu RPC (1983) Laminar origins of spinal projection neurons to periaqueductal gray of the rat. Brain Res 264:118-122
Liu RPC (1986) Spinal neuronal collaterals to the intralaminar thalamic nuclei and periaqueductal gray. Brain Res 365:145-150
Livingstone M, Hubel D (1988) Segregation of form, color, movement, and depth: Anatomy, physiology and perception. Science 240:740-749
Mantyh PW (1982) Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol 206:146-15
Mantyh PW (1983) Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J Neurophysiol 49:567-581
Marchand JE, Hagino N (1983) Afferents to the periaqueductal gray in the rat. A horseradish peroxidase study. Neuroscience 9:95-106
Mark VM, Ervin FR (1969) Stereotaxic surgery for relief of pain. In: Pain and the Neurosurgeon, White JC, Sweet WH, eds. Springfield, IL: Thomas Publishing, pp 843-887
Mayer DJ, Liebeskind JC (1974) Pain reduction by focal electrical stimulation of the brain: An anatomical and behavioral analysis. Brain Res 68:73-93
McCormick DA, Pape H-C (1988) Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature 334:246-248
Melzack R (1975) The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1:277-299
Melzack R, Casey KL (1968) Sensory, motivational and central control determinants of pain. A new conceptual model. In: The Skin Senses, Kenshalo DR, ed. Springfield, IL: Thomas, pp 423-439
Melzack R, Wall PD (1965) Pain mechanisms: A new Theory.Science 150:971-979
Mesulam M-M, Mufson EJ (1982) Insula of the Old World mnkey. Part III. Efferent cortical output. J Comp Neurol 212:38-52
Miletic V, Coffield JA (1989) Responses of neurons in the rat nucleus submedius to noxious and innocuous mechanical cutaneous stimulation. Somatosens Motor Res 6:567-587
Minciacchi D, Bentivoglio M, Molinari M, Kultas-Ilinsky K, Ilinsky IA, Macchi G (1986) Multiple cortical targets of one thalamic nucleus: the projections of the ventral medial nucleus in the cat studied with retrograde tracers. J Comp Neurol 252:106-129
Mosso JA, Kruger L (1973) Receptor catgories represented in spinal trigeminal nucleus caudalis. J Neurophysiol 36:472-488
Müller-Preuss P, Jürgens U (1976) Projections from the 'cingular’ vocalization area in the squirrel monkey. Brain Res 103:29-43
Nashold BS Jr, Wilson WP, Slaughter DG (1969) Sensations evoked by stimulation in the midbrain of man. J Neurosurg 30:14-24
Nishijo H, Yamamoto Y, Ono T, Uwano T, Yamashita J, Yamashima T (1997) Single neuron responses in the monkey anterior cingulate cortex during visual discrimination. Neurosci Lett 227:79-82
Olszewski J (1952) The Thalamus of the Macaca Mullatta, S. Karger, Basel
Perl ER (1987) Pain and nociception. In: Handbook of Physiology Section I: The Nervous System Vol III Sensory Processes Part 2, Brookhart JM, Montcastle VB, eds. American Physiol Soc
Peschanski M, Guilbaud G, Gautron M (1981) Posterior intralaminar region in rat: Neuronal responses to noxious and nonnoxious cutaneous stimuli. Exp Neurol 72:226-238
Peyron R, Garcia-Larrea L, Gregoire M-C, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B (1999) Haemodynamic brain responses to acute pain in humans. Brain 122:1765-1779
Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta analysis. Neurophysiol Clin 30:263-288
Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. (1999) Dissociating pain from its anticipation in the human brain. Science 284:1979-1981
Ploner M, Freund H-J, Schnitzler A (1999) Pain affect without pain sensation in a patient with a postcentral lesion. Pain 81:211-214
Raichle ME, Fiez JA, Videen TO, MacLeod A-MK, Pardo JV, Fox PT, Peterson SE (1994) Practice-related changes in human brain functional anatomy during non-motor learning. Cereb Cor 4:8-26
Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR et al. (1994) Regional cerebral blood flow measured during symptomprovocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatr 51:62-70
Redgrave P, Dean P, Mitchell IJ, Odekunle A, Clark A (1988) The projection from superior colliculus to cuneiform area in the rat. I. Anatomical studies. Exp Brain Res 72:611-625
Richardson DE, Akil H (1977a) Pain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sites. J Neurosurg 47:178-183
Richardson DE, Akil H (1977b) Pain reduction by electrical brain stimulation in man. Part 2: Chronic self-administration in the periventricular gray matter. J Neurosurg 47:184-194
Room P, Russchen FT, Groenewegen HJ, Lohman AHM (1985) Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. J Comp Neurol 242:40-55
Royce GJ (1983a) Cells of origin of corticothalamic projections upon the centromedian and parafascicular nuclei in the cat. Brain Res 258:11-21
Royce GJ (1983b) Cortical neurons with collateral projections to both the caudate nucleus and the centromedian-parafascicular thalamic complex: a fluorescent retrograde double labeling study in the cat. Exp Brain Res 50:157-165
Rydenhag B, Olausson B, Shyu BC, Andersson S (1986) Localized responses in the midsuprasylvian gyrus of the cat following stimulation of the central lateral nucleus in thalamus. Exp Brain Res 62:11-24
Sakata S, Shima F, Kato M, Fukuj M (1988) Effects of thalamic parafascicular stimulation on the periaqueductal gray and adjacent reticular formation neurons. A possible contribution to pain control mechanisms. Brain Res 451:85-96
Sano K (1977) Intralaminar thalamotomy (Thalamolaminotomy) and postero-medial hypothalamotomy in the treatment of intractable pain. Prog Neurol Surg 8:50-103
Saper CB, Loewy AD (1980) Efferent connections of the parabrachial nucleus in the rat. Brain Res 197:291-317
Schlag-Rey M, Schlag J (1984) Visuomotor functions of central thalamus in monkey. I. Unit activity related to spontaneous eye movements. J Neurophysiol 51:1149-1174
Shigenaga Y, Suemune S, Nishimura M, Nishimori T, Sato E, Ishidori H, Yoshida A, Tsuru K, Tsuiki Y, Dateoka W, Nasution ID, Hosoi M (1986) Topographic representation of lower and upper teeth within the trigeminal sensory nuclei of adult cat as demonstrated by the transganglionic transport of horseradish peroxidase. J Comp Neurol 251:299-316
Sikes RW, Vogt BA (1992) Nociceptive neurons in area 24b of rabbit anterior cingulate cortex. J Neurophysiol, 68:1720-1732
Silverman DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA (1997) Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology 112:64-72
Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL (1997) Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol 78:450-460
Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH (1991) Multiple representations of pain in human cerebral cortex. Science 251:1355-1358
Turnbull IM (1972) Bilateral cingulumotomy combined with thalamotomy or mesencephalic tractotomy for pain. Surg Gynecol Obstet Obs 134:958-962
Vaccarino AL, Melzack R (1989) Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain 39:213-219
Vogt BA, Derbyshire SWJ, Jones AKP (1996) Pain processing in four regions of human cingulate cortex localized with coregistered PET and MR imaging. Eur J Neurosci 8:1461-1473
Vogt BA, Gabriel M, Vogt LJ, Poremba A, Jensen EL, Kubota Y, Kang E (1991) Muscarinic receptor binding increases in anterior thalamus and cingulate cortex during discriminative avoidance learning. J Neurosci 11:1508-1514
Vogt BA, Pandya DN (1987) Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262:271-289
Vogt BA, Pandya DN, Rosene DL (1987) Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262:256-270
Vogt BA, Rosene DL, Pandya DN (1979) Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science 204:205-207
Vogt BA, Sikes RW (2000) The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Prog Brain Res, Mayer EA, Saper CB, eds, 122:223-235
Vogt BA, Sikes RW, Vogt LJ (1993) Anterior cingulate cortex and the medial pain system. In: Neurobiology of Cingulate Cortex and Limbic Thalalmus, Vogt BA, Gabriel M, eds, Birkhauser Boston, 313-344
Vogt LJ, Vogt BA, Sikes RE (1992c) The limbic thalamus in rabbit: Architecture, projections to cingulate cortex and distribution of muscarinic acetylcholine, GABAA and opioid receptors. J Comp Neurol, in press
Wenk G, Hepler D, Olton D (1984) Behavior alters the uptake of [3H]choline into acetylcholinergic neurons of the nucleus basalis magnocellular and medial septal area. Behav Brain Res 13:129-138
Willis WD (1985) The Pain System. In: Pain and Headache, Gildenberg PL, ed. Basel: Karger, Vol 8
Willis WD, Kenshalo DR, Leonard RB (1979) The cells of origin of the primate spinothalamic tract. J Comp Neurol 188:543-574
Willis WD, Maunz RA, Foreman RD, Coulter JD (1975) Static and dynamic responses of spinothalamic tract neurons to mechanical stimuli. J Neurophysiol 38:587-600
Wyss JM, Sripanidkulchai K (1984) The topography of the mesencephalic and pontine projections from the cingulate cortex of the rat. Brain Res 293:1-15
Yaksh TL, Al-Rodhan NRF, Jensen TS (1988) Sites of action of opiates in production of analgesia. Prog Brain Res 77:371-394
Yasui Y, Itoh K, Kamiya H, Ino T, Mizuno N (1988) Cingulate gyrus of the cat receives projection fibers from the thalamic region ventral to the ventral border of the ventrobasal complex. J Comp Neurol 274:91-100
Zeki S, Shipp S (1988) The functional logic of cortical connections. Nature 335:311-317
Zhang D, Owens CM, Willis WD (1991) Two forms of inhibition of spinothalamic tract neurons produced by stimulation of the periaqueductal gray and the cerebral cortex. J Neurophysiol 65:1567-1579
Aiko Y, Shima F, Hosokawa S, Kato M, Kitamura K (1987) Altered local cerebral glucose utilization induced by electrical stimulation of the thalamic sensory and parafascicular nuclei in rats. Brain Res 408:47-56
An X, Bandler R, Ongur D, Price JL (1998) Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in Macaque monkeys. J Comp Neurol 401:455-479
Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ III, Willis WD Jr. (1985) Diencephalic mechanisms of pain sensation. Brain Res Rev 9:217-296
Amromin GD, Crue BL Jr, Felsoory A, Todd EM (1975) Bilateral stereotaxic cingulotomy following thoracic rhizotomy, cervical cordotomy, and thalamotomy in a patient with intractable pain: A clinical-pathological study. In: Pain: Research and Treatment, Crue BL, ed. New York: Academic Press
Andersen E (1986) Periaqueductal gray and cerebral cortex modulate responses of medial thalamic neurons to noxious stimulation. Brain Res 375:30-36
Angus-Leppan H, Olausson B, Lambert GA (1990) Modulation of receptive fields of cat nociceptive thalamic neurons by cholinergic mechanisms. Neurosci Abs 16:566
Apkarian AV, Hodge CJ (1989) Primate spinothalamic pathways: I. A quantitative study of the cells of origin of the spinothalamic pathway. J Comp Neurol 288:447-473
Applebaum AE, Beall JE, Foreman RD, Willis WS (1975) Organization and receptive fields of primate spinothalamic tract neurons. J Neurophysiol 38:572-586
Aziz Q, Thompson DG, Ng VWK, Hamdy S, Sarkar S, Brammer MJ, Bullmore ET,, Hobson A, Tracey I Gregory L, Simmons A, Williams SCR (2000) Cortical processing of human somatic and visceral sensation. J Neurosci 20:2657-2663
Ballantine HT, Cassidy WL, Flanagan NB, Marino R Jr (1967) Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 26:488-495
Baleydier C, Mauguiere F (1980) The duality of the cingulate gyrus in monkey: Neuroanatomical study and functional hypothesis. Brain 103:525-554
Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D (1999) Human brain activation under controlled thermal stimulation and habituation to noxious heat: an MRI study. Magn Reson Med 41:1044-1057
Beitz AJ, Mullett MA, Weiner LL (1983) The periaqueductal gray projections to the rat spinal trigeminal, raphe magnus, gigantocellular pars alpha and paragigantocellular nuclei arise from separate neurons. Brain Res 288:307-314
Bench CJ, Frith CD, Grasby M, Friston KJ, Paulesu E, Frackowiack RSJ, Dolan RJ (1993) Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 31:907-922
Boivie J (1979) An anatomical reinvestigation of the termination of the spinothalamic tract in the monkey. J Comp Neurol 186:343-370
Bullitt E (1990) Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 296:517-530
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cog Sci 4:215-222
Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA (2002) The oddball Stroop: A multidimensional cognitive interference task validated with fMRI in individual subjects. Mol Psychiatr, submitted
Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2001) Dorsal anterior cingulate cortex: A role in reward-based decision-making. Proc Natl Acad Sci USA, in press
Bushnell MC, Duncan GH (1989) Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res 78:415-418
Carstens E, Hartung M, Stelzer B, Zimmerman M (1990) Suppression of a hind limb flexion withdrawal reflex by microinjection of glutamate or morphine into the periaqueductal gray in the rat. Pain 43:105-112
Carstens E, Trevino DL (1978) Anatomical and physiological properties of ipsilaterally projecting spinothalamic neurons in the second cervical segment of the cat's spinal cord. J Comp Neurol 182:167-184
Casey KL (1966) Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol 29:727-750
Casey KL (1999) Forebrain mechanisms of nociception and pain: Analysis through imaging. Proc Natl Acad Sci USA 96:7668-7674
Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA (1994) Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 71:802-807
Chudler EH, Anton F, Dubner R, Kenshalo DR Jr (1990) Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: effect of interstimulus interval. J Neurophysiol 63:559-569
Chudler EH, Dong WK, Kawakami Y (1986) Cortical nociceptive responses and behavioral correlates in the monkey. Brain Res 397:47-60
Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH (1994) Distributed processing of pain and vibration by the human brain. J Neurosci 14:4095-4108
Coghill RC, Sang CN, Maisog JM, Ladarola MJ (1999) Pain intensity processing within the human brain. J Neurophysiol 82:1934-1943
Comans PE, Snow PJ (1981) Ascending projections to nucleus parafascicularis of the cat. Brain Res 230:337-341
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Peterson SE (1991) Selective and divided attention during visual discrimination of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11:2383-2402
Corkin S (1980) A prospective study of cingulotomy. In: The Psychosurgery Debate, Valenstein ES, ed. Chap 9, San Francisco: W.H. Freeman and Co.
Craig AD Jr (1990) Nociceptive neurons in the nucleus submedius (Sm) in the medial thalamus of the cat. Pain 40 (Supp 5):S492
Craig AD Jr (1991) Supraspinal pathways and mechanisms relevant to central pain. In: Pain and Central Nervous System Disease: The Central Pain Syndromes, KL Casey, ed. Chap 12, New York: Raven Press, Ltd.
Craig AD Jr (1994) Spinal and supraspinal processing of specific pain and temperature. In: Progress in Pain Research and Management, Boivie J, Hansson P, Lindblom U, eds, IASP Press, 421-437
Craig AD Jr (1995) Supraspinal projections of lamina I neurons. In: Forebrain Areas Involved in Pain Processing. Besson JM, Guilbaud G, Ollat H, eds, John Libbey Eurotext, 13-25.
Craig AD Jr, Burton H (1981) Spinal and medullary lamina I projection to nucleus submedius in medial thalamus: a possible pain center. J Neurophysiol 45:443-466
Craig AD Jr, Hunsley SJ (1991) Morphine enhances the activity of thermoreceptive cold-specific lamina I spinothalamic neurons in the cat. Brain Res 558:93-97
Craig AD Jr., Heppelmann B, Schaible H.-G. (1988) The projection of the medial and posterior articular nerves of the cat’s knee to the spinal cord. J Comp Neurol 276:279-288
Craig AD Jr, Kniffki K-D (1985) Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol (Lond) 365:197-221
Craig AD Jr, Linington AJ, Kniffki K-D (1989) Cells of origin of spinothalamic tract projections to the medial and lateral thalamus in the cat. J Comp Neurol 289:568-585
Cunningham ET, LeVay S (1986) Laminar and synaptic organization of the projection from the thalamic nucleus centralis to primary visual cortex in the cat. J Comp Neurol 254:65-77
Dado RJ, Giesler GJ Jr (1990) Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci 10:2672-2686
Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ (1997) Functional MRI of Pain- and attention-related activations in the human cingulate cortex. J Neurophysiol 77:3370-3380
Derbyshire SWG (2000) Exploring the pain “neuromatrix.” Curr Rev Pain 4:467-477
Derbyshire SWG, Jones AKP, Gyulani F, Clark S, Townsend D, Firestone LL (1997) Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73:431-445
Derbyshire SWG, Vogt BA, Jones AKP (1998) Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118:52-60
DeYoe EA, Van Essen DC (1988) Concurrent processing streams in monkey visual cortex. Trends Neurosci 11:219-226
Donahue RR, LaGraize SC, Fuchs PN (2001) Electrolytic lesion of the anterior cingulate cortex decreases inflammatory but not neuropathic nociceptive behavior in rats. Brain Res 897:131-138
Dong WK, Ryu H, Wagman IH (1978) Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol 41:1592-1613
Dong WK, Salonen LD, Kawakami Y, Shlwaku T, Kaukoranta M, Martin RF (1989) Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res 484:314-324
Dowman R, Schell S (1999) Evidence that the anterior cingulate and supplementary somatosensory cortices generate the pain-related negative difference potential. Clin Neurophysiol 110:2117-2126.
Foltz EL, White LE (1962) Pain “relief” by frontal cingulumotomy. J Neurosurg 19:89-100
Foltz EL, White LE (1968) The role of rostral cingulumotomy in “pain” relief. Int J Neurol 6:353-373
Fuchs PN, Balinsky M, Melzack R (1996) Electrical stimulation of the cingulum bundle and surrounding cortical tissue reduces formalin-test pain in the rat. Brain Res 743:116-123
Gabriel M (1990) Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. In: Progress in Brain Research, Uylings HBM, Van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, eds. New York: Academic Press 85:467-483
Gabriel M (1993) Discriminative avoidance learning: A model system. In: Neurobiology of Cingulate Cortex and Limbic Thalamus, Vogt BA, Gabriel M, eds, Birkhauser Boston, 478-523
Gabriel M, Foster K, Orona E, Saltwick SE, Stanton M (1980) Neuronal activity of cingulate cortex, anteroventral thalamus, and hippocampal formation in discriminative conditioning: Encoding and extraction of the significance of conditional stimuli. Prog Psychobiol Physiol Psychol 9:125-231
Gabriel M, Vogt BA, Kubota Y, Poremba A, Kang E (1991) Training-stage related neuronal plasticity in limbic thalamus and cingulate cortex during learning: A possible key to mnemonic retrieval. Behav Brain Res, in press
Gibbs CM, Powell DA (1991) Single-unit activity in the dorsomedial prefrontal cortex during the expression of discriminative bradycardia in rabbits. Behav Brain Res 43:79-92
Giesler GJ Jr, Yezierski RP, Gerhart KD, Willis WD (1981) Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: Evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol 46:1285-1308.
Gybels JM, Sweet WH (1989) Neurological treatment of persistent pain. Basel: Karger
Hardy JD (1953) Thresholds of pain and reflex contraction as related to noxious stimulation. J Appl Physiol 5:725-739
Hardy SGP (1985) Analgesia elicited by prefrontal stimulation. Brain Res 339:281-284
Hardy SGP, Haigler HJ (1985) Prefrontal influences upon the midbrain: A possible route for pain modulation. Brain Res 339:285-293
Hardy SGP, Leichnetz GR (1981a) Cortical projections to the periaqueductal gray in the monkey: A retrograde and orthograde horseradish peroxidase study. Neurosci Lett 22:97-101
Hardy SGP, Leichnetz GR (1981b) Frontal cortical projections to the periaqueductal gray in the rat: A retrograde and orthograde horseradish peroxidase study. Neurosci Lett 23:13-17
Harmann PA, Carlton SM, Willis WD (1988) Collaterals of spinothalamic tract cells to the periaqueductal gray: a fluorescent double-labeling study in the rat. Brain Res 441:87-97
Hayashi H (1985) Morphology of terminations of small and large myelinated trigeminal primary afferent fibers in the cat. J Comp Neurol 240:71-89
Herkenham M (1979) The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol 183:487-518
Hitchcock ER, Teixeira MJ (1980) A comparison of results from center-median and basal thalamotomies for pain. Surg Neurol 15:341-351
Hoover DB, Jacobowitz DM (1979) Neurochemical and histochemical studies of the effect of a lesion of the nucleus cuneiformis on the cholinergic innervation of discrete areas of the rat brain. Brain Res 170:113-122
Hurt RW, Ballantine HT Jr (1974) Stereotactic anterior cingulate lesions for persistent pain: a report on 68 cases. In: Clinical Neurosurgery, Wilkins RH, ed. Baltimore: Williams and Wilkins
Hylden JLK, Hayashi H, Dubner R, Bennett GJ (1986) Physiology and morphology of the lamina I spinomesencephalic projection. J Comp Neurol 247:505-515
Isaacson LG, Tanaka D Jr (1986) Cholinergic and non-cholinergic projections from the canine pontomesencephalic tegmentum (Ch5 area) to the caudal intralaminar thalamic nuclei. Exp Brain Res 62:179-188
Ishijima B, Yoshimasu N, Fukushima T, Hori T, Sekino H, Sano K (1975) Nociceptive neurons in the human thalamus. Confin Neurol 37:99-106
Jones AKP, Brown WD, Friston KJ, Qi LY, Frackowiak RSJ (1991) Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc R Soc Lond B 244:39-44
Kaelber WW, Mitchell CL, Yarmat AJ, Afifi AK, Lorens SA (1975) Centrum medianum-parafascicularis lesions and reactivity to noxious and non-noxious stimuli. Exper Brain Res 46:282-290
Kayama Y, Takagi M, Ogawa T (1986) Cholinergic influence of the laterodorsal tegmental nucleus on neuronal activity in the rat lateral geniculate nucleus. J Neurophysiol 56:1297-1309
Kenshalo DR Jr, Chudler EH, Anton F, Dubner R (1988) SI nociceptive neurons participate in the encoding process by whih monkeys perceive the intensity of noxious thermal stimuli. Brain Res 454:378-382
Kenshalo DR Jr, Giesler GJ Jr, Leonard RB, Willis WD (1980) Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol 43:1594-1614
Kenshalo DR, Isensee O (1983) Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol 50:1479-1496
Kenshalo DR Jr, Thomas DA, Dubner R (1991) Primary somatosensory cortical lesions reduce the monkeys' ability to discriminate and detect noxious thermal stimulation. Neurosci Abs 17:1206
Kenshalo DR, Willis WD Jr (1991) The role of the cerebral cortex in pain sensation. In: Cerebral Cortex, Peters A, Jones EG, eds. New York: Plenum Press 9:153-212
Kimura H, McGeer PL, Peng JH, McGeer EG (1981) The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol 200:151-201
Koyama T, Kato K, Tanaka YZ, Mikami A (2001) Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neurosci Res 39:412-430
Koyama T, Tanaka YZ, Mikami A (1998) Nociceptive neurons in the macaque anterior cingulate cortex during anticipation of pain. NeuroReport 9:2663-2667
Kumazawa T, Perl ER (1978) Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol 177:417-434
Kupers RC, Vos BPJ, Gybels JM (1988) Stimulation of the nucleus paraventricularis thalami suppresses scratching and biting behavior of arthritic rats and exerts a powerful effect on tests for acute pain. Pain 32:115-125
Kwan CL, Crawley AP, Mikulis DJ, Davis KD (2000) An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85:359-374
La Bars D, Dickenson AH, Besson JM (1979) Diffuse noxious inhibitory controls (DNIC) I. Effects on dorsal horn convergent neurones in the rat. II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6:283-327
Levin B, Duchowny M (1991) Childhood obsessive-compulsive disorder and cingulate epilepsy. Biol Psychiatr 30:1049-1055
Lewin W (1961) Observations on selective leukotomy. J Neurol Neurosurg Psychiat 24:37-44
Light AR, Perl ER (1979) Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186:133-150
Liu RPC (1983) Laminar origins of spinal projection neurons to periaqueductal gray of the rat. Brain Res 264:118-122
Liu RPC (1986) Spinal neuronal collaterals to the intralaminar thalamic nuclei and periaqueductal gray. Brain Res 365:145-150
Livingstone M, Hubel D (1988) Segregation of form, color, movement, and depth: Anatomy, physiology and perception. Science 240:740-749
Mantyh PW (1982) Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J Comp Neurol 206:146-15
Mantyh PW (1983) Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J Neurophysiol 49:567-581
Marchand JE, Hagino N (1983) Afferents to the periaqueductal gray in the rat. A horseradish peroxidase study. Neuroscience 9:95-106
Mark VM, Ervin FR (1969) Stereotaxic surgery for relief of pain. In: Pain and the Neurosurgeon, White JC, Sweet WH, eds. Springfield, IL: Thomas Publishing, pp 843-887
Mayer DJ, Liebeskind JC (1974) Pain reduction by focal electrical stimulation of the brain: An anatomical and behavioral analysis. Brain Res 68:73-93
McCormick DA, Pape H-C (1988) Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature 334:246-248
Melzack R (1975) The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1:277-299
Melzack R, Casey KL (1968) Sensory, motivational and central control determinants of pain. A new conceptual model. In: The Skin Senses, Kenshalo DR, ed. Springfield, IL: Thomas, pp 423-439
Melzack R, Wall PD (1965) Pain mechanisms: A new Theory.Science 150:971-979
Mesulam M-M, Mufson EJ (1982) Insula of the Old World mnkey. Part III. Efferent cortical output. J Comp Neurol 212:38-52
Miletic V, Coffield JA (1989) Responses of neurons in the rat nucleus submedius to noxious and innocuous mechanical cutaneous stimulation. Somatosens Motor Res 6:567-587
Minciacchi D, Bentivoglio M, Molinari M, Kultas-Ilinsky K, Ilinsky IA, Macchi G (1986) Multiple cortical targets of one thalamic nucleus: the projections of the ventral medial nucleus in the cat studied with retrograde tracers. J Comp Neurol 252:106-129
Mosso JA, Kruger L (1973) Receptor catgories represented in spinal trigeminal nucleus caudalis. J Neurophysiol 36:472-488
Müller-Preuss P, Jürgens U (1976) Projections from the 'cingular’ vocalization area in the squirrel monkey. Brain Res 103:29-43
Nashold BS Jr, Wilson WP, Slaughter DG (1969) Sensations evoked by stimulation in the midbrain of man. J Neurosurg 30:14-24
Nishijo H, Yamamoto Y, Ono T, Uwano T, Yamashita J, Yamashima T (1997) Single neuron responses in the monkey anterior cingulate cortex during visual discrimination. Neurosci Lett 227:79-82
Olszewski J (1952) The Thalamus of the Macaca Mullatta, S. Karger, Basel
Perl ER (1987) Pain and nociception. In: Handbook of Physiology Section I: The Nervous System Vol III Sensory Processes Part 2, Brookhart JM, Montcastle VB, eds. American Physiol Soc
Peschanski M, Guilbaud G, Gautron M (1981) Posterior intralaminar region in rat: Neuronal responses to noxious and nonnoxious cutaneous stimuli. Exp Neurol 72:226-238
Peyron R, Garcia-Larrea L, Gregoire M-C, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B (1999) Haemodynamic brain responses to acute pain in humans. Brain 122:1765-1779
Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta analysis. Neurophysiol Clin 30:263-288
Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. (1999) Dissociating pain from its anticipation in the human brain. Science 284:1979-1981
Ploner M, Freund H-J, Schnitzler A (1999) Pain affect without pain sensation in a patient with a postcentral lesion. Pain 81:211-214
Raichle ME, Fiez JA, Videen TO, MacLeod A-MK, Pardo JV, Fox PT, Peterson SE (1994) Practice-related changes in human brain functional anatomy during non-motor learning. Cereb Cor 4:8-26
Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR et al. (1994) Regional cerebral blood flow measured during symptomprovocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatr 51:62-70
Redgrave P, Dean P, Mitchell IJ, Odekunle A, Clark A (1988) The projection from superior colliculus to cuneiform area in the rat. I. Anatomical studies. Exp Brain Res 72:611-625
Richardson DE, Akil H (1977a) Pain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sites. J Neurosurg 47:178-183
Richardson DE, Akil H (1977b) Pain reduction by electrical brain stimulation in man. Part 2: Chronic self-administration in the periventricular gray matter. J Neurosurg 47:184-194
Room P, Russchen FT, Groenewegen HJ, Lohman AHM (1985) Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. J Comp Neurol 242:40-55
Royce GJ (1983a) Cells of origin of corticothalamic projections upon the centromedian and parafascicular nuclei in the cat. Brain Res 258:11-21
Royce GJ (1983b) Cortical neurons with collateral projections to both the caudate nucleus and the centromedian-parafascicular thalamic complex: a fluorescent retrograde double labeling study in the cat. Exp Brain Res 50:157-165
Rydenhag B, Olausson B, Shyu BC, Andersson S (1986) Localized responses in the midsuprasylvian gyrus of the cat following stimulation of the central lateral nucleus in thalamus. Exp Brain Res 62:11-24
Sakata S, Shima F, Kato M, Fukuj M (1988) Effects of thalamic parafascicular stimulation on the periaqueductal gray and adjacent reticular formation neurons. A possible contribution to pain control mechanisms. Brain Res 451:85-96
Sano K (1977) Intralaminar thalamotomy (Thalamolaminotomy) and postero-medial hypothalamotomy in the treatment of intractable pain. Prog Neurol Surg 8:50-103
Saper CB, Loewy AD (1980) Efferent connections of the parabrachial nucleus in the rat. Brain Res 197:291-317
Schlag-Rey M, Schlag J (1984) Visuomotor functions of central thalamus in monkey. I. Unit activity related to spontaneous eye movements. J Neurophysiol 51:1149-1174
Shigenaga Y, Suemune S, Nishimura M, Nishimori T, Sato E, Ishidori H, Yoshida A, Tsuru K, Tsuiki Y, Dateoka W, Nasution ID, Hosoi M (1986) Topographic representation of lower and upper teeth within the trigeminal sensory nuclei of adult cat as demonstrated by the transganglionic transport of horseradish peroxidase. J Comp Neurol 251:299-316
Sikes RW, Vogt BA (1992) Nociceptive neurons in area 24b of rabbit anterior cingulate cortex. J Neurophysiol, 68:1720-1732
Silverman DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA (1997) Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology 112:64-72
Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL (1997) Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol 78:450-460
Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH (1991) Multiple representations of pain in human cerebral cortex. Science 251:1355-1358
Turnbull IM (1972) Bilateral cingulumotomy combined with thalamotomy or mesencephalic tractotomy for pain. Surg Gynecol Obstet Obs 134:958-962
Vaccarino AL, Melzack R (1989) Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain 39:213-219
Vogt BA, Derbyshire SWJ, Jones AKP (1996) Pain processing in four regions of human cingulate cortex localized with coregistered PET and MR imaging. Eur J Neurosci 8:1461-1473
Vogt BA, Gabriel M, Vogt LJ, Poremba A, Jensen EL, Kubota Y, Kang E (1991) Muscarinic receptor binding increases in anterior thalamus and cingulate cortex during discriminative avoidance learning. J Neurosci 11:1508-1514
Vogt BA, Pandya DN (1987) Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262:271-289
Vogt BA, Pandya DN, Rosene DL (1987) Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262:256-270
Vogt BA, Rosene DL, Pandya DN (1979) Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science 204:205-207
Vogt BA, Sikes RW (2000) The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Prog Brain Res, Mayer EA, Saper CB, eds, 122:223-235
Vogt BA, Sikes RW, Vogt LJ (1993) Anterior cingulate cortex and the medial pain system. In: Neurobiology of Cingulate Cortex and Limbic Thalalmus, Vogt BA, Gabriel M, eds, Birkhauser Boston, 313-344
Vogt LJ, Vogt BA, Sikes RE (1992c) The limbic thalamus in rabbit: Architecture, projections to cingulate cortex and distribution of muscarinic acetylcholine, GABAA and opioid receptors. J Comp Neurol, in press
Wenk G, Hepler D, Olton D (1984) Behavior alters the uptake of [3H]choline into acetylcholinergic neurons of the nucleus basalis magnocellular and medial septal area. Behav Brain Res 13:129-138
Willis WD (1985) The Pain System. In: Pain and Headache, Gildenberg PL, ed. Basel: Karger, Vol 8
Willis WD, Kenshalo DR, Leonard RB (1979) The cells of origin of the primate spinothalamic tract. J Comp Neurol 188:543-574
Willis WD, Maunz RA, Foreman RD, Coulter JD (1975) Static and dynamic responses of spinothalamic tract neurons to mechanical stimuli. J Neurophysiol 38:587-600
Wyss JM, Sripanidkulchai K (1984) The topography of the mesencephalic and pontine projections from the cingulate cortex of the rat. Brain Res 293:1-15
Yaksh TL, Al-Rodhan NRF, Jensen TS (1988) Sites of action of opiates in production of analgesia. Prog Brain Res 77:371-394
Yasui Y, Itoh K, Kamiya H, Ino T, Mizuno N (1988) Cingulate gyrus of the cat receives projection fibers from the thalamic region ventral to the ventral border of the ventrobasal complex. J Comp Neurol 274:91-100
Zeki S, Shipp S (1988) The functional logic of cortical connections. Nature 335:311-317
Zhang D, Owens CM, Willis WD (1991) Two forms of inhibition of spinothalamic tract neurons produced by stimulation of the periaqueductal gray and the cerebral cortex. J Neurophysiol 65:1567-1579