|
mo·til·i·ty
\ mō-ˈti-lə-tē \ noun
non·hor·mon·al
/nän-hȯr-ˈmō-nᵊl/ adjective
Useful Blog Posts
External Resources:
|
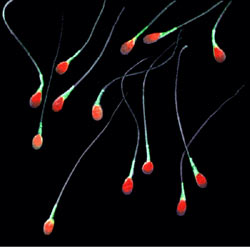
Sperm MotilitySperm motility is the ability of sperm to swim through the female reproductive tract in order to fertilize an egg. The tail of the sperm, the flagellum, is what gives sperm motility. Powered by Adenosine triphosphate (ATP), the tail propels sperm towards its target by whipping back and forth.
However, sperm don’t move in one single straight line. Sperm motility can be classified in different ways, such as straight-moving, zig zag, vibrating, or just non-motile. These different forms of motility move the sperm in different ways. Exposure to different signals changes the guidance mechanism, ultimately guiding the sperm to the egg.
Once the sperm nears the egg, they undergo a process called capacitation. This process is triggered by the presence of calcium, and induces a period of hyperactivation in the sperm. The flagella move with a high curvature and wavelength, propelling the sperm towards the egg. This step also begins destabilizing the acrosomal membrane, which is crucial for fertilization. Any drug that blocks sperm motility could bring about real change to the contraceptive world. These drugs could have a very short onset time, and might only need to be taken a short time before coitus. Also, because sperm demonstrate their motility in the female reproductive tract, women might be able to use this contraceptive as well, bringing true equity to the contraceptive landscape. There are many well-known targets of sperm motility that could be used for development of male contraceptives. MCI has funded work investigating sperm motility targets like the ion channel CatSper, CRISP1, and Na/K ATPase. MCI is also supporting the development of Eppin, who are taking their male contraceptive towards clinical studies. |
Male Reproduction & Contraception
The science behind male reproduction can be challenging, yet it is critical to understand the biology in order to know how the male contraceptives of the future will function. In an effort to make this science more accessible, we have developed a series of primers about male reproduction and contraception:
References
|
Ernesto, J. I., Weigel Muñoz, M., Battistone, M. A., Vasen, G., Martínez-López, P., Orta, G., … Cuasnicú, P. S. (2015). CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. The Journal of Cell Biology, 210(7), 1213–1224. https://doi.org/10.1083/jcb.201412041
O’Rand, M. G., Widgren, E. E., Sivashanmugam, P., Richardson, R. T., Hall, S. H., French, F. S., … & Rao, A. J. (2004). Reversible immunocontraception in male monkeys immunized with Eppin. Science, 306(5699), 1189-1190. Qi, H., Moran, M. M., Navarro, B., Chong, J. A., Krapivinsky, G., Krapivinsky, L., … & Clapham, D. E. (2007). All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proceedings of the National Academy of Sciences, 104(4), 1219-1223. |
Syeda, S. S., Sánchez, G., Hong, K. H., Hawkinson, J. E., Georg, G. I., & Blanco, G. (2018). Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na, K-ATPase α4 Isoform Inhibitors for Male Contraception. Journal of medicinal chemistry, 61(5), 1800-1820.
Hoang, H. D., & Miller, M. A. (2017). Sperm Navigation Mechanisms in the Female Reproductive Tract. In Results and Problems in Cell Differentiation (pp. 241–267). Springer International Publishing. https://doi.org/10.1007/978-3-319-44820-6_9 Pereira, R., Sá, R., Barros, A., & Sousa, M. (2015). Major regulatory mechanisms involved in sperm motility. Asian Journal of Andrology, 0(0), 0. https://doi.org/10.4103/1008-682x.167716 |