|
sper·mat·o·gen·e·sis
/spərmədəˈjenəsəs/ noun
non·hor·mon·al
/nän-hȯr-ˈmō-nᵊl/ adjective
Useful Blog Posts
External Resources:
|
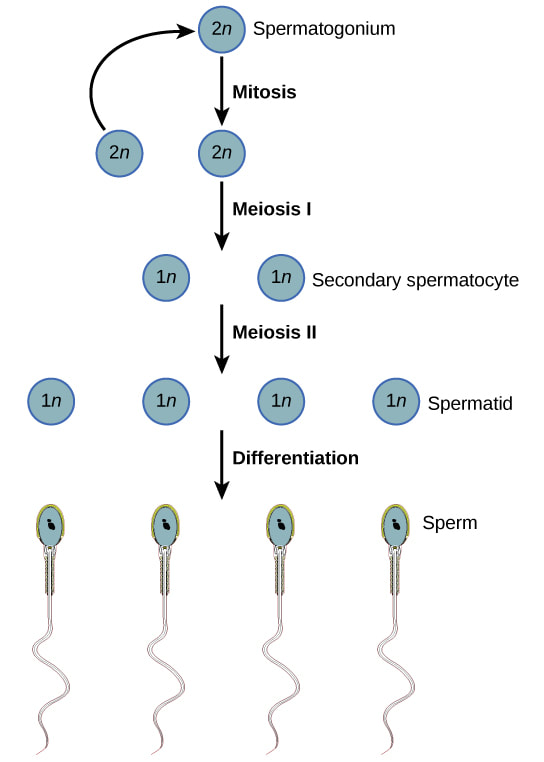
SpermatogenesisSpermatogenesis is the creation of mature sperm from germ cells. Early-stage germ cells, called spermatagonia, reside in the periphery of seminiferous tubules of the testes. This population contains both stem cells and differentiating spermatogonia, which undergo a series of mitotic divisions to amplify the number of cells. Germ cells then enter meiosis, the second phase of spermatogenesis, and are called spermatocytes.
During meiosis, genetic recombination occurs and spermatocytes undergo two divisions which halves the number of chromosomes present in each cell. During spermiogenesis, the final phase of spermatogenesis, haploid germ cells (spermatids) undergo extensive changes and become more like what we think of as traditional sperm, with tails and other structures like the acrosome. Finally, the non-motile spermatozoa are transported to the epididymis. Sperm undergo a maturation process as they transit the epididymis. As a result, they acquire the capacity for forward motility and become capable of fertilization. One way to influence spermatogenesis is through the manipulation of hormone levels. There are ongoing clinical trials focused on creating hormonal male contraceptives, but there are multiple non-hormonal approaches to stop spermatogenesis or interfere with epididymal maturation.
Male contraceptives that stop spermatogenesis would have some specific characteristics, including a potential onset time of 2-3 months. This is because it takes quite some time for sperm to develop after their creation, and even after ceasing spermatogenesis all of the existing sperm in the pipeline would continue to develop. MCI has funded projects in this space, such as Gunda Georg at University of Minnesota and her work on TSSK1/2, Zhibing Zhang at Wayne State University, and some of the Male Contraceptive Initiative Fellows. Other projects at institutions worldwide are focused on diverse approaches to temporarily disrupt spermatogenesis or epididymal maturation so that functional sperm are not produced. |
Male Reproduction & Contraception
The science behind male reproduction can be challenging, yet it is critical to understand the biology in order to know how the male contraceptives of the future will function. In an effort to make this science more accessible, we have developed a series of primers about male reproduction and contraception:
References
|
Xu, B., Hao, Z., Jha, K. N., Zhang, Z., Urekar, C., Digilio, L., … & Herr, J. C. (2008). Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Developmental biology, 319(2), 211-222.
Griswold, M. D. (2016). Spermatogenesis: The Commitment to Meiosis. Physiological Reviews, 96(1), 1–17. https://doi.org/10.1152/physrev.00013.2015 Matzuk, M. M., McKeown, M. R., Filippakopoulos, P., Li, Q., Ma, L., Agno, J. E., … & Knapp, S. (2012). Small-molecule inhibition of BRDT for male contraception. Cell, 150(4), 673-684. Chung, S. S., Wang, X., Roberts, S. S., Griffey, S. M., Reczek, P. R., & Wolgemuth, D. J. (2011). Oral administration of a retinoic acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology, 152(6), 2492-2502. |
Ayoub, R., Page, S. T., Swerdloff, R. S., Liu, P. Y., Amory, J. K., Leung, A., … Wang, C. (2016). Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive. Andrology, 5(2), 278–285. https://doi.org/10.1111/andr.12303
Zitzmann, M., Rohayem, J., Raidt, J., Kliesch, S., Kumar, N., Sitruk-Ware, R., & Nieschlag, E. (2017). Impact of various progestins with or without transdermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: a randomized clinical trial. Andrology, 5(3), 516–526. https://doi.org/10.1111/andr.12328 |