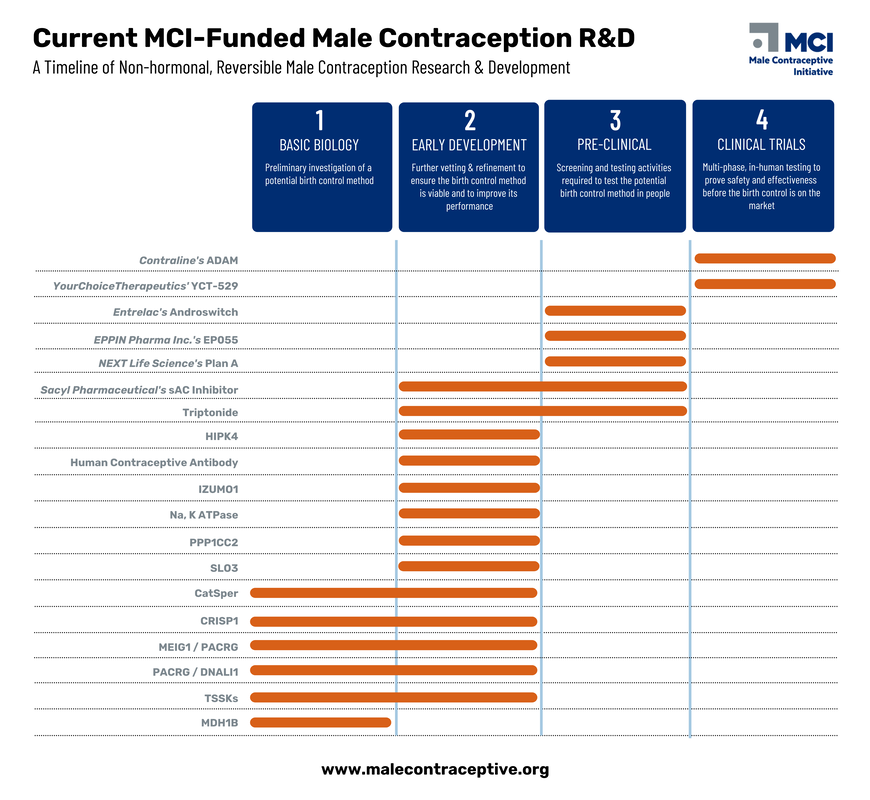
One of the most common questions we receive is when new male birth control options will be available. While it's challenging to predict an exact timeline, we're committed to accelerating the development of innovative solutions.
Discover the promising research projects we're funding and learn about the potential timeline for their development. Stay informed about the latest advancements in male contraception and be among the first to know when new options become available.
Driving Innovation in Male Contraception
Male Contraceptive Initiative is dedicated to advancing the development of non-hormonal, reversible male birth control options. We support a diverse range of research projects across the entire product development pipeline, from early-stage discovery to advanced clinical trials.
Our Commitment:
Explore the Research Initiatives
Discover the exciting work being done by our grantees and the research community at-large as they push the boundaries of male contraception research. Learn about the promising projects that are shaping the future of reproductive health.
Our Commitment:
- Comprehensive support: We provide funding and resources to researchers at all stages of development.
- Diverse portfolio: We support a variety of research approaches to ensure a robust pipeline of options.
- Innovation focus: We prioritize projects that have the potential to make a significant impact.
Explore the Research Initiatives
Discover the exciting work being done by our grantees and the research community at-large as they push the boundaries of male contraception research. Learn about the promising projects that are shaping the future of reproductive health.
(The process, and related timelines, related to evaluating and testing new drugs and therapeutic interventions can be a bit challenging to fully understand. In an effort to simplify this, we have created this primer for your reference.)
Exploring the Potential of Male Contraception
There's a promising pipeline of non-hormonal, reversible male contraceptive targets waiting to be explored. Male Contraceptive Initiative is committed to supporting as many of these promising candidates as possible through our funding activities.
Discover the Pipeline
Visit our grantees page to learn about the specific research projects we're funding. We also maintain a comprehensive database of all identified non-hormonal, reversible male birth control methods. This database is regularly updated to reflect the latest advancements in the field.
For More Information
Explore Calliope from FHI360’s CTI Exchange, a valuable resource for information on the contraceptive development pipeline.
Join Our Research Community
Are you a researcher interested in advancing male contraception? We're eager to collaborate with you. Contact us to discuss potential partnerships and explore funding opportunities.
Discover the Pipeline
Visit our grantees page to learn about the specific research projects we're funding. We also maintain a comprehensive database of all identified non-hormonal, reversible male birth control methods. This database is regularly updated to reflect the latest advancements in the field.
For More Information
Explore Calliope from FHI360’s CTI Exchange, a valuable resource for information on the contraceptive development pipeline.
Join Our Research Community
Are you a researcher interested in advancing male contraception? We're eager to collaborate with you. Contact us to discuss potential partnerships and explore funding opportunities.
Our Research Portfolio: Paving the Way for New Male Contraceptives
Discover the promising research projects we're funding to advance the development of non-hormonal, reversible male birth control options.
- Early-stage targets: Explore innovative approaches in the early stages of development.
- Advanced projects: Support research that is nearing clinical trials.
- Global collaboration: Partner with researchers from around the world.
CatSper
Summary
Catsper1 is a sperm-specific ion channel that plays key roles in sperm functions like hyperactivation. Inhibiting the performance of Catsper could lead to male infertility.
The Science
CatSper is a calcium-selective ion channel confined to the tail of mature sperm. CatSper is required for hyperactivated sperm motility and thus fertilization. CatSper is encoded by four distinct genes, CatSper1-4, which form the pore of the channel. Additional subunits are also required for proper assembly of the channel along a quadrilateral complex from the sperm midpiece to end of the tail. Mutant CatSper genes in humans are associated with male infertility. Small molecule screens have yielded inhibitors of CatSper function which require refining for potency, specificity, and pharmacokinetics. (Learn more here.)
Publications
Hwang JY, Chung JJ. CatSper Calcium Channels: 20 Years On. Physiology (Bethesda). 2023 May 1;38(3):0. doi: 10.1152/physiol.00028.2022. Epub 2022 Dec 13. PMID: 36512352; PMCID: PMC10085559.
Lishko PV, Botchkina IL, Kirichok Y (March 2011). "Progesterone activates the principal Ca2+ channel of human sperm". Nature. 471 (7338): 387–91. Bibcode:2011Natur.471..387L. doi:10.1038/nature09767. PMID 21412339. S2CID 4340309.
Carlson EJ, Francis R, Liu Y, Li P, Lyon M, Santi CM, Hook DJ, Hawkinson JE, Georg GI. Discovery and Characterization of Multiple Classes of Human CatSper Blockers. ChemMedChem. 2022 May 29:e202000499. doi: 10.1002/cmdc.202000499. Epub ahead of print. PMID: 35644882.
Zhao Y, Wang H, Wiesehoefer C, Shah NB, Reetz E, Hwang JY, Huang X, Wang TE, Lishko PV, Davies KM, Wennemuth G, Nicastro D, Chung JJ. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat Commun. 2022 Jun 17;13(1):3439. doi: 10.1038/s41467-022-31050-8. PMID: 35715406; PMCID: PMC9205950.
Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, et al. (January 2007). "All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility". Proceedings of the National Academy of Sciences of the United States of America. 104 (4): 1219–23. doi:10.1073/pnas.0610286104. PMC 1770895. PMID 17227845.
Hwang JY, Wang H, Lu Y, Ikawa M, Chung JJ. C2cd6-encoded CatSperτ targets sperm calcium channel to Ca2+ signaling domains in the flagellar membrane. Cell Rep. 2022 Jan 5:110226. doi: 10.1016/j.celrep.2021.110226. Epub ahead of print. PMID: 34998468.
Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE (January 2011). "A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa". Nature Communications. 2 (1): 153. Bibcode:2011NatCo...2..153C. doi:10.1038/ncomms1153. PMC 3999383. PMID 21224844.
Lishko PV, Botchkina IL, Kirichok Y (March 2011). "Progesterone activates the principal Ca2+ channel of human sperm". Nature. 471 (7338): 387–91. Bibcode:2011Natur.471..387L. doi:10.1038/nature09767. PMID 21412339. S2CID 4340309.
Catsper1 is a sperm-specific ion channel that plays key roles in sperm functions like hyperactivation. Inhibiting the performance of Catsper could lead to male infertility.
The Science
CatSper is a calcium-selective ion channel confined to the tail of mature sperm. CatSper is required for hyperactivated sperm motility and thus fertilization. CatSper is encoded by four distinct genes, CatSper1-4, which form the pore of the channel. Additional subunits are also required for proper assembly of the channel along a quadrilateral complex from the sperm midpiece to end of the tail. Mutant CatSper genes in humans are associated with male infertility. Small molecule screens have yielded inhibitors of CatSper function which require refining for potency, specificity, and pharmacokinetics. (Learn more here.)
Publications
Hwang JY, Chung JJ. CatSper Calcium Channels: 20 Years On. Physiology (Bethesda). 2023 May 1;38(3):0. doi: 10.1152/physiol.00028.2022. Epub 2022 Dec 13. PMID: 36512352; PMCID: PMC10085559.
Lishko PV, Botchkina IL, Kirichok Y (March 2011). "Progesterone activates the principal Ca2+ channel of human sperm". Nature. 471 (7338): 387–91. Bibcode:2011Natur.471..387L. doi:10.1038/nature09767. PMID 21412339. S2CID 4340309.
Carlson EJ, Francis R, Liu Y, Li P, Lyon M, Santi CM, Hook DJ, Hawkinson JE, Georg GI. Discovery and Characterization of Multiple Classes of Human CatSper Blockers. ChemMedChem. 2022 May 29:e202000499. doi: 10.1002/cmdc.202000499. Epub ahead of print. PMID: 35644882.
Zhao Y, Wang H, Wiesehoefer C, Shah NB, Reetz E, Hwang JY, Huang X, Wang TE, Lishko PV, Davies KM, Wennemuth G, Nicastro D, Chung JJ. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat Commun. 2022 Jun 17;13(1):3439. doi: 10.1038/s41467-022-31050-8. PMID: 35715406; PMCID: PMC9205950.
Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, et al. (January 2007). "All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility". Proceedings of the National Academy of Sciences of the United States of America. 104 (4): 1219–23. doi:10.1073/pnas.0610286104. PMC 1770895. PMID 17227845.
Hwang JY, Wang H, Lu Y, Ikawa M, Chung JJ. C2cd6-encoded CatSperτ targets sperm calcium channel to Ca2+ signaling domains in the flagellar membrane. Cell Rep. 2022 Jan 5:110226. doi: 10.1016/j.celrep.2021.110226. Epub ahead of print. PMID: 34998468.
Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE (January 2011). "A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa". Nature Communications. 2 (1): 153. Bibcode:2011NatCo...2..153C. doi:10.1038/ncomms1153. PMC 3999383. PMID 21224844.
Lishko PV, Botchkina IL, Kirichok Y (March 2011). "Progesterone activates the principal Ca2+ channel of human sperm". Nature. 471 (7338): 387–91. Bibcode:2011Natur.471..387L. doi:10.1038/nature09767. PMID 21412339. S2CID 4340309.
|
MCI Grantee & Fellow Conversations: Jae Yeon Hwang
|
Single Molecule Puts Sperm on Track
|
Contraline's ADAM™
ADAM™
Summary
This is a hydrogel injected directly into a man’s vas deferens which provides a physical blockage and prevents sperm from exiting the body. It is intended to effectively be a reversible vasectomy.
The Science
ADAM™ is a hydrogel designed to block sperm from traveling through the vas deferens without affecting physical sensation or ejaculation. The hydrogel is intended to be injected directly into a man’s vas deferens in an outpatient setting using local anesthesia using a procedure very similar to a no-scalpel vasectomy. Sperm that are blocked naturally degrade before being absorbed into the vas deferens. At the end of its proposed 2-year lifespan, the hydrogel liquifies and sperm begins to flow naturally once again. ADAM™ is in human studies before market availability, and they are actively recruiting participants. (Learn more here.)
Publications
Matsumoto NM, Chiartas TG, Paysour BR, Barry TJ, Ott LE, Tropsha Y, Eisenfrats KS. Preclinical development of a novel injectable hydrogel for vas-occlusion. Contraception. 2025 Feb 10:110839. doi: 10.1016/j.contraception.2025.110839. Epub ahead of print. PMID: 39938674.
Khourdaji I, Zillioux J, Eisenfrats K, Foley D, Smith R. The future of male contraception: a fertile ground. Transl Androl Urol. 2018 May;7(Suppl 2):S220-S235. doi: 10.21037/tau.2018.03.23. PMID: 29928620; PMCID: PMC5989114.
This is a hydrogel injected directly into a man’s vas deferens which provides a physical blockage and prevents sperm from exiting the body. It is intended to effectively be a reversible vasectomy.
The Science
ADAM™ is a hydrogel designed to block sperm from traveling through the vas deferens without affecting physical sensation or ejaculation. The hydrogel is intended to be injected directly into a man’s vas deferens in an outpatient setting using local anesthesia using a procedure very similar to a no-scalpel vasectomy. Sperm that are blocked naturally degrade before being absorbed into the vas deferens. At the end of its proposed 2-year lifespan, the hydrogel liquifies and sperm begins to flow naturally once again. ADAM™ is in human studies before market availability, and they are actively recruiting participants. (Learn more here.)
Publications
Matsumoto NM, Chiartas TG, Paysour BR, Barry TJ, Ott LE, Tropsha Y, Eisenfrats KS. Preclinical development of a novel injectable hydrogel for vas-occlusion. Contraception. 2025 Feb 10:110839. doi: 10.1016/j.contraception.2025.110839. Epub ahead of print. PMID: 39938674.
Khourdaji I, Zillioux J, Eisenfrats K, Foley D, Smith R. The future of male contraception: a fertile ground. Transl Androl Urol. 2018 May;7(Suppl 2):S220-S235. doi: 10.21037/tau.2018.03.23. PMID: 29928620; PMCID: PMC5989114.
|
MCI Grantee & Fellow Conversations: Kevin Eisenfrats
|
Contraline Explainer Video
|
CRISP1
Cysteine-rich Secretory Protein 1
Summary
Studies have found that the presence of CRISP1 leads to a reduced ability of sperm to fuse to eggs.
The Science
The CRISP family of mammals has 4 members: CRISP1, CRISP2, CRISP3 and CRISP4. Evidence suggests that CRISP proteins have evolved to perform a variety of functions based on their domain structure. In both humans and mice, CRISP1 is secreted in the lumen of the epididymal tubule and is found on the surface of gametes in distinct locations between the two models since in the mouse CRISP1 is located in the dorsal region of the acrosome while in the human CRISP1 is located in the post-acrosomal compartment. Two populations of CRISP1 proteins are bound to gametes, a majority fraction with a labile association and a minority fraction but with solid binding. The disengagement of the labile fraction seems to be necessary for capacitation, which suggested that CRISP1 could be involved in preventing too early initiation of capacitation during transit and epididymal storage.
Publications
Li T, Guo H. Overexpression of PD-L1 causes germ cell failure and infertility via CRISP1/PD-L1 interaction in mouse epididymis. Zygote. 2024 Jun;32(3):224-229. doi: 10.1017/S0967199424000157. Epub 2024 Jun 3. PMID: 38828560.
Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev Biol. 2008 Aug 1;320(1):12-8. doi: 10.1016/j.ydbio.2008.03.015. Epub 2008 Mar 20. PMID: 18571638; PMCID: PMC2603034.
Cohen DJ, Maldera JA, Vasen G, Ernesto JI, Muñoz MW, Battistone MA, Cuasnicú PS. Epididymal protein CRISP1 plays different roles during the fertilization process. J Androl. 2011 Nov-Dec;32(6):672-8. doi: 10.2164/jandrol.110.012922. Epub 2011 Mar 25. PMID: 21441424.
Ernesto JI, Weigel Muñoz M, Battistone MA, Vasen G, Martínez-López P, Orta G, Figueiras-Fierro D, De la Vega-Beltran JL, Moreno IA, Guidobaldi HA, Giojalas L, Darszon A, Cohen DJ, Cuasnicú PS. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J Cell Biol. 2015 Sep 28;210(7):1213-24. doi: 10.1083/jcb.201412041. PMID: 26416967; PMCID: PMC4586743.
Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007 Nov;77(5):848-54. doi: 10.1095/biolreprod.107.061788. Epub 2007 Aug 1. PMID: 17671267.
Studies have found that the presence of CRISP1 leads to a reduced ability of sperm to fuse to eggs.
The Science
The CRISP family of mammals has 4 members: CRISP1, CRISP2, CRISP3 and CRISP4. Evidence suggests that CRISP proteins have evolved to perform a variety of functions based on their domain structure. In both humans and mice, CRISP1 is secreted in the lumen of the epididymal tubule and is found on the surface of gametes in distinct locations between the two models since in the mouse CRISP1 is located in the dorsal region of the acrosome while in the human CRISP1 is located in the post-acrosomal compartment. Two populations of CRISP1 proteins are bound to gametes, a majority fraction with a labile association and a minority fraction but with solid binding. The disengagement of the labile fraction seems to be necessary for capacitation, which suggested that CRISP1 could be involved in preventing too early initiation of capacitation during transit and epididymal storage.
Publications
Li T, Guo H. Overexpression of PD-L1 causes germ cell failure and infertility via CRISP1/PD-L1 interaction in mouse epididymis. Zygote. 2024 Jun;32(3):224-229. doi: 10.1017/S0967199424000157. Epub 2024 Jun 3. PMID: 38828560.
Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev Biol. 2008 Aug 1;320(1):12-8. doi: 10.1016/j.ydbio.2008.03.015. Epub 2008 Mar 20. PMID: 18571638; PMCID: PMC2603034.
Cohen DJ, Maldera JA, Vasen G, Ernesto JI, Muñoz MW, Battistone MA, Cuasnicú PS. Epididymal protein CRISP1 plays different roles during the fertilization process. J Androl. 2011 Nov-Dec;32(6):672-8. doi: 10.2164/jandrol.110.012922. Epub 2011 Mar 25. PMID: 21441424.
Ernesto JI, Weigel Muñoz M, Battistone MA, Vasen G, Martínez-López P, Orta G, Figueiras-Fierro D, De la Vega-Beltran JL, Moreno IA, Guidobaldi HA, Giojalas L, Darszon A, Cohen DJ, Cuasnicú PS. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J Cell Biol. 2015 Sep 28;210(7):1213-24. doi: 10.1083/jcb.201412041. PMID: 26416967; PMCID: PMC4586743.
Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007 Nov;77(5):848-54. doi: 10.1095/biolreprod.107.061788. Epub 2007 Aug 1. PMID: 17671267.
EPPIN
Epididymal Protease Inhibitor
Summary
This protein is critical for sperm movement; preventing its proper functioning could prevent sperm from being able to exit the male body.
The Science
Eppin is a sperm surface protein that binds semenogelin (SEMG1) and is critical for the protection of sperm from the enzymatic degradation of the semen coagulum, which frees the spermatozoa for motility and capacitation. Eppin is the contraceptive target for a small organic compound that binds Eppin and inhibits sperm motility. Blocking Eppin has been also shown to inhibit the motility required for fertilization by decreasing the sperm pH and reducing calcium levels. Eppin is in pre-clinical development. (Learn more here.)
Publications
Wang Z, Widgren EE, Richardson RT, Orand MG. Eppin: a molecular strategy for male contraception. Soc Reprod Fertil Suppl. 2007;65:535-42. PMID: 17644992.
O'Rand MG, Silva EJ, Hamil KG. Non-hormonal male contraception: A review and development of an Eppin based contraceptive. Pharmacol Ther. 2016 Jan;157:105-11. doi: 10.1016/j.pharmthera.2015.11.004. Epub 2015 Nov 22. PMID: 26593445; PMCID: PMC4703502.
Silva EJR, Hamil KG, et al. (2012) Characterization of EPPIN's semenogelin I binding site: A contraceptive drug target. Biol Repro 87(3):56, 1-8.
O'Rand MG, Widgren EE, et al. (2006) Eppin: an effective target for male contraception. Mol Cell Endocrinol 250:157-162.
O'Rand MG, Hamil K, Adevai T, Zelinski M. (2018). Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PLoS ONE 13(4): e0195953.
Thirumalai A, Amory JK. Emerging approaches to male contraception. Fertil Steril. 2021 Jun;115(6):1369-1376. doi: 10.1016/j.fertnstert.2021.03.047. Epub 2021 Apr 27. PMID: 33931201; PMCID: PMC8169637.
This protein is critical for sperm movement; preventing its proper functioning could prevent sperm from being able to exit the male body.
The Science
Eppin is a sperm surface protein that binds semenogelin (SEMG1) and is critical for the protection of sperm from the enzymatic degradation of the semen coagulum, which frees the spermatozoa for motility and capacitation. Eppin is the contraceptive target for a small organic compound that binds Eppin and inhibits sperm motility. Blocking Eppin has been also shown to inhibit the motility required for fertilization by decreasing the sperm pH and reducing calcium levels. Eppin is in pre-clinical development. (Learn more here.)
Publications
Wang Z, Widgren EE, Richardson RT, Orand MG. Eppin: a molecular strategy for male contraception. Soc Reprod Fertil Suppl. 2007;65:535-42. PMID: 17644992.
O'Rand MG, Silva EJ, Hamil KG. Non-hormonal male contraception: A review and development of an Eppin based contraceptive. Pharmacol Ther. 2016 Jan;157:105-11. doi: 10.1016/j.pharmthera.2015.11.004. Epub 2015 Nov 22. PMID: 26593445; PMCID: PMC4703502.
Silva EJR, Hamil KG, et al. (2012) Characterization of EPPIN's semenogelin I binding site: A contraceptive drug target. Biol Repro 87(3):56, 1-8.
O'Rand MG, Widgren EE, et al. (2006) Eppin: an effective target for male contraception. Mol Cell Endocrinol 250:157-162.
O'Rand MG, Hamil K, Adevai T, Zelinski M. (2018). Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PLoS ONE 13(4): e0195953.
Thirumalai A, Amory JK. Emerging approaches to male contraception. Fertil Steril. 2021 Jun;115(6):1369-1376. doi: 10.1016/j.fertnstert.2021.03.047. Epub 2021 Apr 27. PMID: 33931201; PMCID: PMC8169637.
Durham company trying to develop birth control pill for men
GAPDHS
Glyceraldehyde 3-phosphate Dehydrogenase-S
Summary
This enzyme helps “feed” sperm and is necessary for them to have the energy to swim. Preventing its function could cause male infertility.
The Science
Glyceraldehyde 3-phosphate dehydrogenase-S (GAPDHS) is a sperm specific enzyme, and is part of the metabolic pathway required to convert glucose into ATP (source of energy for sperm). By binding GAPDHS and inhibiting glycolysis, sperm motility is impaired, thus leading to infertility. (Learn more here.)
Publications
Miki K, Qu W, et al. (2004) Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 101:16501-16506.
Cooper TG, Yeung CH (Jun 1999). "Approaches to post-testicular contraception". Asian Journal of Andrology. 1 (1–2): 29–36.
Danshina PV, Qu W, Temple BR, Rojas RJ, Miley MJ, Machius M, Betts L, O'Brien DA. Structural analyses to identify selective inhibitors of glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme. Mol Hum Reprod. 2016 Jun;22(6):410-26. doi: 10.1093/molehr/gaw016. Epub 2016 Feb 26. PMID: 26921398; PMCID: PMC4884916.
Bunch DO, Welch JE, Magyar PL, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S protein distribution during mouse spermatogenesis. Biol Reprod. 1998 Mar;58(3):834-41. doi: 10.1095/biolreprod58.3.834. PMID: 9510974.
This enzyme helps “feed” sperm and is necessary for them to have the energy to swim. Preventing its function could cause male infertility.
The Science
Glyceraldehyde 3-phosphate dehydrogenase-S (GAPDHS) is a sperm specific enzyme, and is part of the metabolic pathway required to convert glucose into ATP (source of energy for sperm). By binding GAPDHS and inhibiting glycolysis, sperm motility is impaired, thus leading to infertility. (Learn more here.)
Publications
Miki K, Qu W, et al. (2004) Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 101:16501-16506.
Cooper TG, Yeung CH (Jun 1999). "Approaches to post-testicular contraception". Asian Journal of Andrology. 1 (1–2): 29–36.
Danshina PV, Qu W, Temple BR, Rojas RJ, Miley MJ, Machius M, Betts L, O'Brien DA. Structural analyses to identify selective inhibitors of glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme. Mol Hum Reprod. 2016 Jun;22(6):410-26. doi: 10.1093/molehr/gaw016. Epub 2016 Feb 26. PMID: 26921398; PMCID: PMC4884916.
Bunch DO, Welch JE, Magyar PL, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S protein distribution during mouse spermatogenesis. Biol Reprod. 1998 Mar;58(3):834-41. doi: 10.1095/biolreprod58.3.834. PMID: 9510974.
CHEM 407 - Glycolysis - 6 - Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH)
HCA
Human Contraceptive Antibody
Summary
HCA is an antibody that attaches to a glycoprotein to agglutinate and immobilize sperm.
The Science
The Human Contraceptive Antibody (HCA) is an IgG1 mAb antibody that has the potential to agglutinate and immobilize sperm through attaching to CD52g, a glycoprotein that is found in the male reproductive tract and on sperm. Sperm sticking together and losing their mobility would lead to male infertility. HCA is currently being developed as a contraceptive delivered to women through vaginal films and as a couple’s product contraceptive incorporated into lubricants. Other work with HCA intends to incorporate it into a multipurpose prevention technology (MPT), which could provide protection against both pregnancy and sexually transmitted infections.
Publications
Anderson, D. J., Politch, J. A., Cone, R. A., Zeitlin, L., Lai, S. K., Santangelo, P. J., Moench, T. R., & Whaley, K. J. (2020). Engineering monoclonal antibody-based contraception and multipurpose prevention technologies†. Biology of reproduction, 103(2), 275–285. https://doi.org/10.1093/biolre/ioaa096
Baldeon-Vaca, G., Marathe, J. G., Politch, J. A., Mausser, E., Pudney, J., Doud, J., Nador, E., Zeitlin, L., Pauly, M., Moench, T. R., Brennan, M., Whaley, K. J., & Anderson, D. J. (2021). Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine, 69, 103478. https://doi.org/10.1016/j.ebiom.2021.103478
Politch, J. A., Cu-Uvin, S., Moench, T. R., Tashima, K. T., Marathe, J. G., Guthrie, K. M., Cabral, H., Nyhuis, T., Brennan, M., Zeitlin, L., Spiegel, H. M. L., Mayer, K. H., Whaley, K. J., & Anderson, D. J. (2021). Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A Phase I randomized trial. PLoS medicine, 18(2), e1003495. https://doi.org/10.1371/journal.pmed.1003495
HCA is an antibody that attaches to a glycoprotein to agglutinate and immobilize sperm.
The Science
The Human Contraceptive Antibody (HCA) is an IgG1 mAb antibody that has the potential to agglutinate and immobilize sperm through attaching to CD52g, a glycoprotein that is found in the male reproductive tract and on sperm. Sperm sticking together and losing their mobility would lead to male infertility. HCA is currently being developed as a contraceptive delivered to women through vaginal films and as a couple’s product contraceptive incorporated into lubricants. Other work with HCA intends to incorporate it into a multipurpose prevention technology (MPT), which could provide protection against both pregnancy and sexually transmitted infections.
Publications
Anderson, D. J., Politch, J. A., Cone, R. A., Zeitlin, L., Lai, S. K., Santangelo, P. J., Moench, T. R., & Whaley, K. J. (2020). Engineering monoclonal antibody-based contraception and multipurpose prevention technologies†. Biology of reproduction, 103(2), 275–285. https://doi.org/10.1093/biolre/ioaa096
Baldeon-Vaca, G., Marathe, J. G., Politch, J. A., Mausser, E., Pudney, J., Doud, J., Nador, E., Zeitlin, L., Pauly, M., Moench, T. R., Brennan, M., Whaley, K. J., & Anderson, D. J. (2021). Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine, 69, 103478. https://doi.org/10.1016/j.ebiom.2021.103478
Politch, J. A., Cu-Uvin, S., Moench, T. R., Tashima, K. T., Marathe, J. G., Guthrie, K. M., Cabral, H., Nyhuis, T., Brennan, M., Zeitlin, L., Spiegel, H. M. L., Mayer, K. H., Whaley, K. J., & Anderson, D. J. (2021). Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A Phase I randomized trial. PLoS medicine, 18(2), e1003495. https://doi.org/10.1371/journal.pmed.1003495
HIPK4
Homeodomain-interacting Protein Kinase 4
Summary
HIPK4 is an essential regulator of spermiogenesis and sperm head shaping.
The Science
The homeodomain-interacting protein kinase 4 (HIPK4) is a protein coding gene that plays a role in later stages of sperm maturation and is another potential nonhormonal target under development. Hipk4 mutant sperm have reduced oocyte binding and are incompetent for in vitro fertilization, but they can still produce viable offspring via intracytoplasmic sperm injection. Optical and electron microscopy of HIPK4-null male germ cells reveals defects in the filamentous actin (F-actin)-scaffolded acroplaxome during spermatid elongation, creating abnormal head morphologies in mature spermatozoa, resulting in male infertility. HIPK4 knockout mice have impaired spermatogenesis but are otherwise healthy.
Publications
Crapster, J. A., Rack, P. G., Hellmann, Z. J., Le, A. D., Adams, C. M., Leib, R. D., Elias, J. E., Perrino, J., Behr, B., Li, Y., Lin, J., Zeng, H., & Chen, J. K. (2020). HIPK4 is essential for murine spermiogenesis. eLife, 9, e50209. https://doi.org/10.7554/eLife.50209.
He Q, Shi J, Sun H, An J, Huang Y, Sheikh MS. Characterization of Human Homeodomain-interacting Protein Kinase 4 (HIPK4) as a Unique Member of the HIPK Family. Mol Cell Pharmacol. 2010;2(2):61-68.
Long JE, Lee MS, Blithe DL. Male Contraceptive Development: Update on Novel Hormonal and Nonhormonal Methods. Clin Chem. 2019 Jan;65(1):153-160.
HIPK4 is an essential regulator of spermiogenesis and sperm head shaping.
The Science
The homeodomain-interacting protein kinase 4 (HIPK4) is a protein coding gene that plays a role in later stages of sperm maturation and is another potential nonhormonal target under development. Hipk4 mutant sperm have reduced oocyte binding and are incompetent for in vitro fertilization, but they can still produce viable offspring via intracytoplasmic sperm injection. Optical and electron microscopy of HIPK4-null male germ cells reveals defects in the filamentous actin (F-actin)-scaffolded acroplaxome during spermatid elongation, creating abnormal head morphologies in mature spermatozoa, resulting in male infertility. HIPK4 knockout mice have impaired spermatogenesis but are otherwise healthy.
Publications
Crapster, J. A., Rack, P. G., Hellmann, Z. J., Le, A. D., Adams, C. M., Leib, R. D., Elias, J. E., Perrino, J., Behr, B., Li, Y., Lin, J., Zeng, H., & Chen, J. K. (2020). HIPK4 is essential for murine spermiogenesis. eLife, 9, e50209. https://doi.org/10.7554/eLife.50209.
He Q, Shi J, Sun H, An J, Huang Y, Sheikh MS. Characterization of Human Homeodomain-interacting Protein Kinase 4 (HIPK4) as a Unique Member of the HIPK Family. Mol Cell Pharmacol. 2010;2(2):61-68.
Long JE, Lee MS, Blithe DL. Male Contraceptive Development: Update on Novel Hormonal and Nonhormonal Methods. Clin Chem. 2019 Jan;65(1):153-160.
|
Novel Contraceptives Targeting Sperm Development and Function
|
Protein Kinases: Cell Signaling and Phosphorylation
|
ISFI
In-situ Forming Implant
Summary
This long-acting, biodegradable implant injected just below the skin’s surface is capable of delivering a male contraceptive over a sustained period of time and then be naturally absorbed by the body after the drug is exhausted.
The Science
Researchers are developing a long-acting (LA) biodegradable polymeric solid implant (PSI) fabricated using a new process that allows for the fabrication of solid implants that can have different shapes and sizes, accommodate high drug payloads, and provide sustained drug release over several months.The release kinetics of drugscan be fine-tuned by varying drug-loading concentration, the ratio of polymer (poly(lactic-co-glycolic acid), PLGA) to solvent (N-methyl-2-pyrrolidone, NMP) and polymer (PLGA) molecular weight in the precursor solution. The research findings indicate that the versatility of this technology makes it an attractive drug delivery platform that could be adapted to a number of potential drug-based male contraceptives.
Publications
Benhabbour, S. R., Kovarova, M., Jones, C., Copeland, D. J., Shrivastava, R., Swanson, M. D., Sykes, C., Ho, P. T., Cottrell, M. L., Sridharan, A., Fix, S. M., Thayer, O., Long, J. M., Hazuda, D. J., Dayton, P. A., Mumper, R. J., Kashuba, A. D. M., & Victor Garcia, J. (2019). Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nature Communications, 10(1), 4324. https://doi.org/10.1038/s41467-019-12141-5
Maturavongsadit, P., Paravyan, G., Kovarova, M., Garcia, J. V., & Benhabbour, S. R. (2021). A new engineering process of biodegradable polymeric solid implants for ultra-long-acting drug delivery. International Journal of Pharmaceutics: X, 3, 100068. https://doi.org/10.1016/j.ijpx.2020.100068
Howard SA, Benhabbour SR. Non-Hormonal Contraception. J Clin Med. 2023 Jul 20;12(14):4791. doi: 10.3390/jcm12144791. PMID: 37510905; PMCID: PMC10381146.
This long-acting, biodegradable implant injected just below the skin’s surface is capable of delivering a male contraceptive over a sustained period of time and then be naturally absorbed by the body after the drug is exhausted.
The Science
Researchers are developing a long-acting (LA) biodegradable polymeric solid implant (PSI) fabricated using a new process that allows for the fabrication of solid implants that can have different shapes and sizes, accommodate high drug payloads, and provide sustained drug release over several months.The release kinetics of drugscan be fine-tuned by varying drug-loading concentration, the ratio of polymer (poly(lactic-co-glycolic acid), PLGA) to solvent (N-methyl-2-pyrrolidone, NMP) and polymer (PLGA) molecular weight in the precursor solution. The research findings indicate that the versatility of this technology makes it an attractive drug delivery platform that could be adapted to a number of potential drug-based male contraceptives.
Publications
Benhabbour, S. R., Kovarova, M., Jones, C., Copeland, D. J., Shrivastava, R., Swanson, M. D., Sykes, C., Ho, P. T., Cottrell, M. L., Sridharan, A., Fix, S. M., Thayer, O., Long, J. M., Hazuda, D. J., Dayton, P. A., Mumper, R. J., Kashuba, A. D. M., & Victor Garcia, J. (2019). Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nature Communications, 10(1), 4324. https://doi.org/10.1038/s41467-019-12141-5
Maturavongsadit, P., Paravyan, G., Kovarova, M., Garcia, J. V., & Benhabbour, S. R. (2021). A new engineering process of biodegradable polymeric solid implants for ultra-long-acting drug delivery. International Journal of Pharmaceutics: X, 3, 100068. https://doi.org/10.1016/j.ijpx.2020.100068
Howard SA, Benhabbour SR. Non-Hormonal Contraception. J Clin Med. 2023 Jul 20;12(14):4791. doi: 10.3390/jcm12144791. PMID: 37510905; PMCID: PMC10381146.
MCI’s Lemonade Stand: Male Contraceptive Delivery Modalities
IZUMO1
Izumo Sperm-Egg Fusion 1
Summary
The protein Izumo is sperm-specific, named for a Japanese shrine dedicated to marriage, and is essential for sperm-egg plasma membrane binding and fusion.
The Science
Izumo sperm-egg fusion 1 (IZUMO1) is a protein that is encoded by the IZUMO1 gene in humans. In mammalian fertilisation, IZUMO1 binds to its egg receptor counterpart, Juno, to facilitate recognition and fusion of the gametes. The final step before sperm and egg combine to become a zygote, disrupting the interaction between these two proteins is a potential pathway towards new male or female contraceptives.
Publications
Hernández-Falcó M, Sáez-Espinosa P, López-Botella A, Aizpurua J, Gómez-Torres MJ. The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review. Int J Mol Sci. 2022 Apr 1;23(7):3929. doi: 10.3390/ijms23073929. PMID: 35409288; PMCID: PMC8999778.
Young SA, Aitken J, Baker MA. Phosphorylation of Izumo1 and Its Role in Male Infertility. Asian J Androl. 2015 Sep-Oct;17(5):708-10. doi: 10.4103/1008-682X.156119. PMID: 25994654; PMCID: PMC4577576.
Gómez-Torres MJ, Hernández-Falcó M, López-Botella A, Huerta-Retamal N, Sáez-Espinosa P. IZUMO1 Receptor Localization during Hyaluronic Acid Selection in Human Spermatozoa. Biomedicines. 2023 Oct 24;11(11):2872. doi: 10.3390/biomedicines11112872. PMID: 38001873; PMCID: PMC10669769.
Stepanenko N, Wolk O, Bianchi E, Wright GJ, Schachter-Safrai N, Makedonski K, Ouro A, Ben-Meir A, Buganim Y, Goldblum A. In silico Docking Analysis for Blocking JUNO-IZUMO1 Interaction Identifies Two Small Molecules that Block in vitro Fertilization. Front Cell Dev Biol. 2022 Apr 5;10:824629. doi: 10.3389/fcell.2022.824629. PMID: 35478965; PMCID: PMC9037035.
Bianchi, Enrica, et al. "Juno is the egg Izumo receptor and is essential for mammalian fertilization." Nature 508.7497 (2014): 483.
Inoue N, Ikawa M, Isotani A, Okabe M (2005). "The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs". Nature. 434 (7030): 234–8.
Saito T, Wada I, Inoue N. Sperm IZUMO1-Dependent Gamete Fusion Influences Male Fertility in Mice. Int J Mol Sci. 2019 Sep 27;20(19):4809.
The protein Izumo is sperm-specific, named for a Japanese shrine dedicated to marriage, and is essential for sperm-egg plasma membrane binding and fusion.
The Science
Izumo sperm-egg fusion 1 (IZUMO1) is a protein that is encoded by the IZUMO1 gene in humans. In mammalian fertilisation, IZUMO1 binds to its egg receptor counterpart, Juno, to facilitate recognition and fusion of the gametes. The final step before sperm and egg combine to become a zygote, disrupting the interaction between these two proteins is a potential pathway towards new male or female contraceptives.
Publications
Hernández-Falcó M, Sáez-Espinosa P, López-Botella A, Aizpurua J, Gómez-Torres MJ. The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review. Int J Mol Sci. 2022 Apr 1;23(7):3929. doi: 10.3390/ijms23073929. PMID: 35409288; PMCID: PMC8999778.
Young SA, Aitken J, Baker MA. Phosphorylation of Izumo1 and Its Role in Male Infertility. Asian J Androl. 2015 Sep-Oct;17(5):708-10. doi: 10.4103/1008-682X.156119. PMID: 25994654; PMCID: PMC4577576.
Gómez-Torres MJ, Hernández-Falcó M, López-Botella A, Huerta-Retamal N, Sáez-Espinosa P. IZUMO1 Receptor Localization during Hyaluronic Acid Selection in Human Spermatozoa. Biomedicines. 2023 Oct 24;11(11):2872. doi: 10.3390/biomedicines11112872. PMID: 38001873; PMCID: PMC10669769.
Stepanenko N, Wolk O, Bianchi E, Wright GJ, Schachter-Safrai N, Makedonski K, Ouro A, Ben-Meir A, Buganim Y, Goldblum A. In silico Docking Analysis for Blocking JUNO-IZUMO1 Interaction Identifies Two Small Molecules that Block in vitro Fertilization. Front Cell Dev Biol. 2022 Apr 5;10:824629. doi: 10.3389/fcell.2022.824629. PMID: 35478965; PMCID: PMC9037035.
Bianchi, Enrica, et al. "Juno is the egg Izumo receptor and is essential for mammalian fertilization." Nature 508.7497 (2014): 483.
Inoue N, Ikawa M, Isotani A, Okabe M (2005). "The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs". Nature. 434 (7030): 234–8.
Saito T, Wada I, Inoue N. Sperm IZUMO1-Dependent Gamete Fusion Influences Male Fertility in Mice. Int J Mol Sci. 2019 Sep 27;20(19):4809.
|
Juno: A molecule for life - Sanger Institute
|
A Molecule for Life
|
LDHC
Lactate Dehydrogenase C
Summary
Reduced or absent LDHC protein expression or activity may be associated with reduced sperm motility in a man's semen.
The Science
Experiments with infertile LDHC-null male mice point towards LDHC playing an essential role in maintenance of the processes of glycolysis and ATP production in the flagellum that are required for male fertility and sperm function. In LDHC-null sperm glucose consumption was disrupted, but the NAD:NADH ratio and pyruvate levels were unchanged, and pyruvate was rapidly metabolized to lactate. Moreover, the metabolic disorder induced by treatment with the lactate dehydrogenase (LDH) inhibitor sodium oxamate was different from that caused by lack of LDHC. This supported the conclusion that LDHA [lactate dehydrogenase A], an LDH isozyme present in the principal piece of the flagellum, is responsible for the residual LDH activity in sperm lacking LDHC, but suggested that LDHC has an additional role in the maintenance of energy metabolism in sperm. (Learn more here.)
Publications
Esmaeilpour T, Zarei MR, Bahmanpour S, Aliabadi E, Hosseini A, Jaberipour M. Effect of follicular fluid and platelet-activating factor on lactate dehydrogenase C expression in human asthenozoospermic samples. Iran J Med Sci. 2014 Jan;39(1):20-8. PMID: 24453390. PMCID: PMC3895891.
Chang JJ, Peng JP, Yang Y, Wang JL, Xu L. Study on the antifertility effects of the plasmid DNA vaccine expressing partial brLDH-C4'. Reproduction. 2006 Jan;131(1):183-92. doi: 10.1530/rep.1.00262. PMID: 16388021.
Teng X, Emmett MJ, Lazar MA, Goldberg E, Rabinowitz JD. Lactate Dehydrogenase C Produces S-2-Hydroxyglutarate in Mouse Testis. ACS Chem Biol. 2016 Sep 16;11(9):2420-7.
Reduced or absent LDHC protein expression or activity may be associated with reduced sperm motility in a man's semen.
The Science
Experiments with infertile LDHC-null male mice point towards LDHC playing an essential role in maintenance of the processes of glycolysis and ATP production in the flagellum that are required for male fertility and sperm function. In LDHC-null sperm glucose consumption was disrupted, but the NAD:NADH ratio and pyruvate levels were unchanged, and pyruvate was rapidly metabolized to lactate. Moreover, the metabolic disorder induced by treatment with the lactate dehydrogenase (LDH) inhibitor sodium oxamate was different from that caused by lack of LDHC. This supported the conclusion that LDHA [lactate dehydrogenase A], an LDH isozyme present in the principal piece of the flagellum, is responsible for the residual LDH activity in sperm lacking LDHC, but suggested that LDHC has an additional role in the maintenance of energy metabolism in sperm. (Learn more here.)
Publications
Esmaeilpour T, Zarei MR, Bahmanpour S, Aliabadi E, Hosseini A, Jaberipour M. Effect of follicular fluid and platelet-activating factor on lactate dehydrogenase C expression in human asthenozoospermic samples. Iran J Med Sci. 2014 Jan;39(1):20-8. PMID: 24453390. PMCID: PMC3895891.
Chang JJ, Peng JP, Yang Y, Wang JL, Xu L. Study on the antifertility effects of the plasmid DNA vaccine expressing partial brLDH-C4'. Reproduction. 2006 Jan;131(1):183-92. doi: 10.1530/rep.1.00262. PMID: 16388021.
Teng X, Emmett MJ, Lazar MA, Goldberg E, Rabinowitz JD. Lactate Dehydrogenase C Produces S-2-Hydroxyglutarate in Mouse Testis. ACS Chem Biol. 2016 Sep 16;11(9):2420-7.
Lactate Dehydrogenase (LDH) | Biochemistry, Lab, and Clinical significanc
MDH1B
Malate Dehydrogenase 1B
Summary
Inhibiting this enzyme has been shown to prevent sperm from fertilizing an egg, and decrease sperm motility.
The Science
Malate dehydrogenase (MDH) catalyzes the conversion of oxaloacetate and malate. It is a rather ubiquitous enzyme, for which several isoforms have been identified, differing in their subcellular localization and their specificity for the cofactor NAD or NADP. Two major MDH isozymes (MDH-A and MDH-B) are present in sperm, and might be targeted for contraception.
Publications
Haberman A, Peterson CN. Genetics of MDH in humans. Essays Biochem. 2024 Oct 3;68(2):107-119. doi: 10.1042/EBC20230078. PMID: 39037390.
Musrati RA, Kollárová M, Mernik N, Mikulásová D. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998 Sep;17(3):193-210. PMID: 9834842.
Breininger E, Dubois D, Pereyra VE, Rodriguez PC, Satorre MM, Cetica PD. Participation of phosphofructokinase, malate dehydrogenase and isocitrate dehydrogenase in capacitation and acrosome reaction of boar spermatozoa. Reprod Domest Anim. 2017 Oct;52(5):731-740. doi: 10.1111/rda.12973. Epub 2017 Apr 10. PMID: 28397297.
Matsuzawa T, Sawada H. Histochemical changes in rat sperm malate dehydrogenase activity during passage through epididymis. Endocrinol Jpn. 1987 Apr;34(2):231-5. doi: 10.1507/endocrj1954.34.231. PMID: 3622391.
Inhibiting this enzyme has been shown to prevent sperm from fertilizing an egg, and decrease sperm motility.
The Science
Malate dehydrogenase (MDH) catalyzes the conversion of oxaloacetate and malate. It is a rather ubiquitous enzyme, for which several isoforms have been identified, differing in their subcellular localization and their specificity for the cofactor NAD or NADP. Two major MDH isozymes (MDH-A and MDH-B) are present in sperm, and might be targeted for contraception.
Publications
Haberman A, Peterson CN. Genetics of MDH in humans. Essays Biochem. 2024 Oct 3;68(2):107-119. doi: 10.1042/EBC20230078. PMID: 39037390.
Musrati RA, Kollárová M, Mernik N, Mikulásová D. Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys. 1998 Sep;17(3):193-210. PMID: 9834842.
Breininger E, Dubois D, Pereyra VE, Rodriguez PC, Satorre MM, Cetica PD. Participation of phosphofructokinase, malate dehydrogenase and isocitrate dehydrogenase in capacitation and acrosome reaction of boar spermatozoa. Reprod Domest Anim. 2017 Oct;52(5):731-740. doi: 10.1111/rda.12973. Epub 2017 Apr 10. PMID: 28397297.
Matsuzawa T, Sawada H. Histochemical changes in rat sperm malate dehydrogenase activity during passage through epididymis. Endocrinol Jpn. 1987 Apr;34(2):231-5. doi: 10.1507/endocrj1954.34.231. PMID: 3622391.
MEIG1 / PACRG
Meiosis-expressed Gene 1 / Parkin Co-regulated Gene
Summary
The pairing of MEIG1 and PACRG is necessary to help sperm develop their tails (i.e., flagella) in order to swim.
The Science
A key event in the process of spermiogenesis is the formation of the flagella, which enables sperm to reach eggs for fertilization. Yeast two-hybrid studies revealed that meiosis-expressed gene 1 (MEIG1) and Parkin co-regulated gene (PACRG) interact, and that sperm-associated antigen 16, which encodes an axoneme central apparatus protein, is also a binding partner of MEIG1. In spermatocytes of wild-type mice, MEIG1 is expressed in the whole germ cell bodies, but the protein migrates to the manchette, a unique structure at the base of elongating spermatid that directs formation of the flagella. Mice deficient in MEIG1 are infertile as a result of impaired spermatogenesis.
Publications
Hasse T, Zhang Z, Huang YM. In silico discovery of potential inhibitors targeting the MEIG1-PACRG complex for male contraceptive development. bioRxiv [Preprint]. 2024 Dec 20:2024.12.16.628759. doi: 10.1101/2024.12.16.628759. PMID: 39763986; PMCID: PMC11702573.
Yap YT, Li W, Huang Q, Zhou Q, Zhang D, Sheng Y, Mladenovic-Lucas L, Yee SP, Orwig KE, Granneman JG, Williams DC Jr, Hess RA, Toure A, Zhang Z. DNALI1 interacts with the MEIG1/PACRG complex within the manchette and is required for proper sperm flagellum assembly in mice. Elife. 2023 Apr 21;12:e79620. doi: 10.7554/eLife.79620. PMID: 37083624; PMCID: PMC10185345.
Yap YT, Shi L, Zhang D, Huang Q, Siddika F, Wang Z, Li W, Zhang Z. MEIG1 determines the manchette localization of IFT20 and IFT88, two intraflagellar transport components in male germ cells. Dev Biol. 2022 May;485:50-60. doi: 10.1016/j.ydbio.2022.03.001. Epub 2022 Mar 4. PMID: 35257720.
Wei Li, Qian Huang, Ling Zhang, Hong Liu, David Zhang, Shuo Yuan, Yitian Yap, Wei Qu, Rita Shiang, Shizheng Song, Rex A. Hess, Zhibing Zhang. A single amino acid mutation in the mouse MEIG1 protein disrupts a cargo transport system necessary for sperm formation. Journal of Biological Chemistry, 2021, 101312, ISSN 0021-9258, https://doi.org/10.1016/j.jbc.2021.101312.
Li W, Tang W, Teves ME, Zhang Z, Zhang L, Li H, Archer KJ, Peterson DL, Williams DC Jr, Strauss JF 3rd, Zhang Z. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development. 2015 Mar 1;142(5):921-30. doi: 10.1242/dev.119834. PMID: 25715396; PMCID: PMC4352978.
Zhang Z, Shen X, Gude DR, Wilkinson BM, Justice MJ, Flickinger CJ, Herr JC, Eddy EM, Strauss JF 3rd. MEIG1 is essential for spermiogenesis in mice. Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17055-60. doi: 10.1073/pnas.0906414106. Epub 2009 Sep 17. PMID: 19805151; PMCID: PMC2746124.
Li W, Walavalkar NM, Buchwald WA, Teves ME, Zhang L, Liu H, Bilinovich S, Peterson DL, Strauss JF 3rd, Williams DC Jr, Zhang Z. Dissecting the structural basis of MEIG1 interaction with PACRG. Sci Rep. 2016 Jan 4;6:18278. doi: 10.1038/srep18278. PMID: 26726850; PMCID: PMC4698733.
The pairing of MEIG1 and PACRG is necessary to help sperm develop their tails (i.e., flagella) in order to swim.
The Science
A key event in the process of spermiogenesis is the formation of the flagella, which enables sperm to reach eggs for fertilization. Yeast two-hybrid studies revealed that meiosis-expressed gene 1 (MEIG1) and Parkin co-regulated gene (PACRG) interact, and that sperm-associated antigen 16, which encodes an axoneme central apparatus protein, is also a binding partner of MEIG1. In spermatocytes of wild-type mice, MEIG1 is expressed in the whole germ cell bodies, but the protein migrates to the manchette, a unique structure at the base of elongating spermatid that directs formation of the flagella. Mice deficient in MEIG1 are infertile as a result of impaired spermatogenesis.
Publications
Hasse T, Zhang Z, Huang YM. In silico discovery of potential inhibitors targeting the MEIG1-PACRG complex for male contraceptive development. bioRxiv [Preprint]. 2024 Dec 20:2024.12.16.628759. doi: 10.1101/2024.12.16.628759. PMID: 39763986; PMCID: PMC11702573.
Yap YT, Li W, Huang Q, Zhou Q, Zhang D, Sheng Y, Mladenovic-Lucas L, Yee SP, Orwig KE, Granneman JG, Williams DC Jr, Hess RA, Toure A, Zhang Z. DNALI1 interacts with the MEIG1/PACRG complex within the manchette and is required for proper sperm flagellum assembly in mice. Elife. 2023 Apr 21;12:e79620. doi: 10.7554/eLife.79620. PMID: 37083624; PMCID: PMC10185345.
Yap YT, Shi L, Zhang D, Huang Q, Siddika F, Wang Z, Li W, Zhang Z. MEIG1 determines the manchette localization of IFT20 and IFT88, two intraflagellar transport components in male germ cells. Dev Biol. 2022 May;485:50-60. doi: 10.1016/j.ydbio.2022.03.001. Epub 2022 Mar 4. PMID: 35257720.
Wei Li, Qian Huang, Ling Zhang, Hong Liu, David Zhang, Shuo Yuan, Yitian Yap, Wei Qu, Rita Shiang, Shizheng Song, Rex A. Hess, Zhibing Zhang. A single amino acid mutation in the mouse MEIG1 protein disrupts a cargo transport system necessary for sperm formation. Journal of Biological Chemistry, 2021, 101312, ISSN 0021-9258, https://doi.org/10.1016/j.jbc.2021.101312.
Li W, Tang W, Teves ME, Zhang Z, Zhang L, Li H, Archer KJ, Peterson DL, Williams DC Jr, Strauss JF 3rd, Zhang Z. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development. 2015 Mar 1;142(5):921-30. doi: 10.1242/dev.119834. PMID: 25715396; PMCID: PMC4352978.
Zhang Z, Shen X, Gude DR, Wilkinson BM, Justice MJ, Flickinger CJ, Herr JC, Eddy EM, Strauss JF 3rd. MEIG1 is essential for spermiogenesis in mice. Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17055-60. doi: 10.1073/pnas.0906414106. Epub 2009 Sep 17. PMID: 19805151; PMCID: PMC2746124.
Li W, Walavalkar NM, Buchwald WA, Teves ME, Zhang L, Liu H, Bilinovich S, Peterson DL, Strauss JF 3rd, Williams DC Jr, Zhang Z. Dissecting the structural basis of MEIG1 interaction with PACRG. Sci Rep. 2016 Jan 4;6:18278. doi: 10.1038/srep18278. PMID: 26726850; PMCID: PMC4698733.
The Sodium-Potassium Pump
Na,K-ATPase
Sodium, Potassium, ATPase (ATP1A4)
Summary
This sperm-specific isoform of a sodium-potassium ATPase has been shown to be critical to sperm motility: inhibiting it leads to sperm incapable of swimming.
The Science
Regulation of ion balance in spermatozoa has been shown to be essential for sperm motility and fertility. Control of intracellular ion levels requires the function of distinct ion-transport mechanisms at the cell plasma membrane. Active Na(+) and K(+) exchange in sperm is under the control of the Na,K-ATPase, and the sperm-specific isoform Na,K-ATPase α4 is essential for sperm fertility. Deletion of α4 results in severe reduction in sperm motility, a failure of in-vitro fertilization, and reduction in hyperactivation typical of sperm capacitation.
Publications
Clausen MV, Hilbers F and Poulsen H (2017) The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 8:371. doi: 10.3389/fphys.2017.00371
Li Z and Langhans SA (2015) Transcriptional regulators of Na,K-ATPase subunits. Front. Cell Dev. Biol. 3:66. doi: 10.3389/fcell.2015.00066
Milewski D, James PF. Alpha4 Na,K-ATPase Localization and Expression Are Dynamic Aspects of Spermatogenesis and in Sperm Incubated Under Capacitating Conditions. Int J Mol Sci. 2025 Feb 20;26(5):1817. doi: 10.3390/ijms26051817. PMID: 40076443.
Numata S, McDermott JP, Blanco G. Genetic Ablation of Na,K-ATPase α4 Results in Sperm Energetic Defects. Front Cell Dev Biol. 2022 May 26;10:911056. doi: 10.3389/fcell.2022.911056. PMID: 35693932; PMCID: PMC9178190.
Jimenez T, McDermott JP, Sánchez G, Blanco G. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):644-9. doi: 10.1073/pnas.1016902108. Epub 2010 Dec 27. PMID: 21187400; PMCID: PMC3021039.
Cui X, Xie Z. Protein Interaction and Na/K-ATPase-Mediated Signal Transduction. Molecules. 2017 Jun 14;22(6):990. doi: 10.3390/molecules22060990. PMID: 28613263; PMCID: PMC6152704.
Geering K. Na,K-ATPase. Curr Opin Nephrol Hypertens. 1997 Sep;6(5):434-9. doi: 10.1097/00041552-199709000-00005. PMID: 9327201.
This sperm-specific isoform of a sodium-potassium ATPase has been shown to be critical to sperm motility: inhibiting it leads to sperm incapable of swimming.
The Science
Regulation of ion balance in spermatozoa has been shown to be essential for sperm motility and fertility. Control of intracellular ion levels requires the function of distinct ion-transport mechanisms at the cell plasma membrane. Active Na(+) and K(+) exchange in sperm is under the control of the Na,K-ATPase, and the sperm-specific isoform Na,K-ATPase α4 is essential for sperm fertility. Deletion of α4 results in severe reduction in sperm motility, a failure of in-vitro fertilization, and reduction in hyperactivation typical of sperm capacitation.
Publications
Clausen MV, Hilbers F and Poulsen H (2017) The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 8:371. doi: 10.3389/fphys.2017.00371
Li Z and Langhans SA (2015) Transcriptional regulators of Na,K-ATPase subunits. Front. Cell Dev. Biol. 3:66. doi: 10.3389/fcell.2015.00066
Milewski D, James PF. Alpha4 Na,K-ATPase Localization and Expression Are Dynamic Aspects of Spermatogenesis and in Sperm Incubated Under Capacitating Conditions. Int J Mol Sci. 2025 Feb 20;26(5):1817. doi: 10.3390/ijms26051817. PMID: 40076443.
Numata S, McDermott JP, Blanco G. Genetic Ablation of Na,K-ATPase α4 Results in Sperm Energetic Defects. Front Cell Dev Biol. 2022 May 26;10:911056. doi: 10.3389/fcell.2022.911056. PMID: 35693932; PMCID: PMC9178190.
Jimenez T, McDermott JP, Sánchez G, Blanco G. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):644-9. doi: 10.1073/pnas.1016902108. Epub 2010 Dec 27. PMID: 21187400; PMCID: PMC3021039.
Cui X, Xie Z. Protein Interaction and Na/K-ATPase-Mediated Signal Transduction. Molecules. 2017 Jun 14;22(6):990. doi: 10.3390/molecules22060990. PMID: 28613263; PMCID: PMC6152704.
Geering K. Na,K-ATPase. Curr Opin Nephrol Hypertens. 1997 Sep;6(5):434-9. doi: 10.1097/00041552-199709000-00005. PMID: 9327201.
|
Sodium-potassium ATPase: Active Transport
|
The Sodium-Potassium Pump
|
PACRG / DNALI1
Parkin Co-regulated Gene / Dynein Axonemal Light Intermediate Chain 1
Summary
This pairing of two sperm-expressed proteins is presumed to be necessary for sperm to develop flagella and therefore be able to swim.
The Science
Dnali1 is strongly expressed in spermatids and is also detected in spermatocytes. The gene product, DNALI1 is localized to the manchette, and is a cytoskeletal motor protein that moves along microtubules in cells.. The pairing of DNALI1 with PACRG is presumed to be responsible for the maturation of the sperm tail (i.e., flagella), and mice lacking DNALI1 are infertile, demonstrating low sperm counts with abnormal sperm morphology.
Publications
Hu HY, Wei TY, Feng ZK, Li SJ, Zhao R, Yi XL, Hu TL, Zhao H, Li CX, Liu ZG. Novel Biallelic DNAH1 Variations Cause Multiple Morphological Abnormalities of the Sperm Flagella. DNA Cell Biol. 2021 Jun;40(6):833-840. doi: 10.1089/dna.2021.0097. Epub 2021 May 14. PMID: 33989052.
Lorenzetti D, Bishop CE, Justice MJ. Deletion of the Parkin coregulated gene causes male sterility in the quaking(viable) mouse mutant. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8402-7. doi: 10.1073/pnas.0401832101. Epub 2004 May 17. PMID: 15148410; PMCID: PMC420406.
Rashid S, Breckle R, Hupe M, Geisler S, Doerwald N, Neesen J. The murine Dnali1 gene encodes a flagellar protein that interacts with the cytoplasmic dynein heavy chain 1. Mol Reprod Dev. 2006 Jun;73(6):784-94. doi: 10.1002/mrd.20475. PMID: 16496424.
This pairing of two sperm-expressed proteins is presumed to be necessary for sperm to develop flagella and therefore be able to swim.
The Science
Dnali1 is strongly expressed in spermatids and is also detected in spermatocytes. The gene product, DNALI1 is localized to the manchette, and is a cytoskeletal motor protein that moves along microtubules in cells.. The pairing of DNALI1 with PACRG is presumed to be responsible for the maturation of the sperm tail (i.e., flagella), and mice lacking DNALI1 are infertile, demonstrating low sperm counts with abnormal sperm morphology.
Publications
Hu HY, Wei TY, Feng ZK, Li SJ, Zhao R, Yi XL, Hu TL, Zhao H, Li CX, Liu ZG. Novel Biallelic DNAH1 Variations Cause Multiple Morphological Abnormalities of the Sperm Flagella. DNA Cell Biol. 2021 Jun;40(6):833-840. doi: 10.1089/dna.2021.0097. Epub 2021 May 14. PMID: 33989052.
Lorenzetti D, Bishop CE, Justice MJ. Deletion of the Parkin coregulated gene causes male sterility in the quaking(viable) mouse mutant. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8402-7. doi: 10.1073/pnas.0401832101. Epub 2004 May 17. PMID: 15148410; PMCID: PMC420406.
Rashid S, Breckle R, Hupe M, Geisler S, Doerwald N, Neesen J. The murine Dnali1 gene encodes a flagellar protein that interacts with the cytoplasmic dynein heavy chain 1. Mol Reprod Dev. 2006 Jun;73(6):784-94. doi: 10.1002/mrd.20475. PMID: 16496424.
PPP1CC2
Protein Phosphatase 1 Catalytic Subunit Gamma 2
Summary
PPP1CC2 is an isoform found in sperm and contributes to spermatogenesis.
The Science
PPP1CC2 is an isoform that comes from a family of phosphatases found in sperm. Phosphatases are proteins that remove phosphate groups from other proteins, changing their activity and function. This isoform contributes to spermatogenesis through a wide range of interactions with testis and sperm proteins including AKAP4, which is also required for fertility. Selectively disrupting interactions between PPP1CC2 and its partners may cause male infertility.
Publications
MacLeod, G., Shang, P., Booth, G. T., Mastropaolo, L. A., Manafpoursakha, N., Vogl, A. W., & Varmuza, S. (2013). PPP1CC2 can form a kinase/phosphatase complex with the testis-specific proteins TSSK1 and TSKS in the mouse testis. Reproduction (Cambridge, England), 147(1), 1–12. https://doi.org/10.1530/REP-13-0224
Sinha, N., Pilder, S., & Vijayaraghavan, S. (2012). Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PloS one, 7(10), e47623. https://doi.org/10.1371/journal.pone.0047623
Silva, J. V., Yoon, S., De Bock, P. J., Goltsev, A. V., Gevaert, K., Mendes, J. F., & Fardilha, M. (2017). Construction and analysis of a human testis/sperm-enriched interaction network: Unraveling the PPP1CC2 interactome. Biochimica et biophysica acta. General subjects, 1861(2), 375–385. https://doi.org/10.1016/j.bbagen.2016.11.041
Silva JV, Freitas MJ, Fardilha M. Phosphoprotein phosphatase 1 complexes in spermatogenesis. Curr Mol Pharmacol. 2014;7(2):136-46. doi: 10.2174/1874467208666150126154222. PMID: 25620225.
PPP1CC2 is an isoform found in sperm and contributes to spermatogenesis.
The Science
PPP1CC2 is an isoform that comes from a family of phosphatases found in sperm. Phosphatases are proteins that remove phosphate groups from other proteins, changing their activity and function. This isoform contributes to spermatogenesis through a wide range of interactions with testis and sperm proteins including AKAP4, which is also required for fertility. Selectively disrupting interactions between PPP1CC2 and its partners may cause male infertility.
Publications
MacLeod, G., Shang, P., Booth, G. T., Mastropaolo, L. A., Manafpoursakha, N., Vogl, A. W., & Varmuza, S. (2013). PPP1CC2 can form a kinase/phosphatase complex with the testis-specific proteins TSSK1 and TSKS in the mouse testis. Reproduction (Cambridge, England), 147(1), 1–12. https://doi.org/10.1530/REP-13-0224
Sinha, N., Pilder, S., & Vijayaraghavan, S. (2012). Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PloS one, 7(10), e47623. https://doi.org/10.1371/journal.pone.0047623
Silva, J. V., Yoon, S., De Bock, P. J., Goltsev, A. V., Gevaert, K., Mendes, J. F., & Fardilha, M. (2017). Construction and analysis of a human testis/sperm-enriched interaction network: Unraveling the PPP1CC2 interactome. Biochimica et biophysica acta. General subjects, 1861(2), 375–385. https://doi.org/10.1016/j.bbagen.2016.11.041
Silva JV, Freitas MJ, Fardilha M. Phosphoprotein phosphatase 1 complexes in spermatogenesis. Curr Mol Pharmacol. 2014;7(2):136-46. doi: 10.2174/1874467208666150126154222. PMID: 25620225.
Anne Bertolotti (MRC LMB) 1: A Historical Perspective on Protein Phosphatases
RAR⍺
Retinoic Acid Receptor α
Summary
Retinoic Acid Receptor Alpha (RAR⍺) is a protein that interacts with retinoic acid, which is critical for the formation of sperm.
The Science
Retinoid signaling is transduced by 2 families of nuclear receptors, retinoic acid receptor (RAR) and retinoid X receptor (RXR), which form RXR/RAR heterodimers. In the presence of retinoic acid, the complex undergoes a conformational change that initiates transcription and allows for gene expression. It has been validated in studies as a viable male contraceptive target, and molecules binding RAR⍺ have been developed as potential male contraceptive agents that require further optimization and testing. (Learn more here.)
Publications
Mannowetz, N., Chung, S.S.W., Maitra, S. et al. Targeting the retinoid signaling pathway with YCT-529 for effective and reversible oral contraception in mice and primates. Commun Med 5, 68 (2025). https://doi.org/10.1038/s43856-025-00752-7
Shi R, Wolgemuth DJ, Georg GI. Development of the retinoic acid receptor alpha-specific antagonist YCT-529 for male contraception: A brief review. Contraception. 2025 Jan 3:110809. doi: 10.1016/j.contraception.2024.110809. Epub ahead of print. PMID: 39756562.
Md Abdullah Al Noman, Jillian L Kyzer, Sanny S W Chung, Debra J Wolgemuth, Gunda I Georg, Retinoic acid receptor antagonists for male contraception: current status†, Biology of Reproduction, Volume 103, Issue 2, August 2020, Pages 390–399
Kam RK, Deng Y, Chen Y, Zhao H (March 2012). "Retinoic acid synthesis and functions in early embryonic development". Cell & Bioscience. 2 (1): 11.
Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008 Jun;29(4):465-93.
Retinoic Acid Receptor Alpha (RAR⍺) is a protein that interacts with retinoic acid, which is critical for the formation of sperm.
The Science
Retinoid signaling is transduced by 2 families of nuclear receptors, retinoic acid receptor (RAR) and retinoid X receptor (RXR), which form RXR/RAR heterodimers. In the presence of retinoic acid, the complex undergoes a conformational change that initiates transcription and allows for gene expression. It has been validated in studies as a viable male contraceptive target, and molecules binding RAR⍺ have been developed as potential male contraceptive agents that require further optimization and testing. (Learn more here.)
Publications
Mannowetz, N., Chung, S.S.W., Maitra, S. et al. Targeting the retinoid signaling pathway with YCT-529 for effective and reversible oral contraception in mice and primates. Commun Med 5, 68 (2025). https://doi.org/10.1038/s43856-025-00752-7
Shi R, Wolgemuth DJ, Georg GI. Development of the retinoic acid receptor alpha-specific antagonist YCT-529 for male contraception: A brief review. Contraception. 2025 Jan 3:110809. doi: 10.1016/j.contraception.2024.110809. Epub ahead of print. PMID: 39756562.
Md Abdullah Al Noman, Jillian L Kyzer, Sanny S W Chung, Debra J Wolgemuth, Gunda I Georg, Retinoic acid receptor antagonists for male contraception: current status†, Biology of Reproduction, Volume 103, Issue 2, August 2020, Pages 390–399
Kam RK, Deng Y, Chen Y, Zhao H (March 2012). "Retinoic acid synthesis and functions in early embryonic development". Cell & Bioscience. 2 (1): 11.
Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008 Jun;29(4):465-93.
|
MCI Grantee & Fellow Conversations: Md Abdullah Al Noman
|
Retinoic Acid Signaling Pathway
|
British Men Trial Contraceptive Pill | Petrie Hosken | Dr Grunda Georg
|
A birth Control Pill For Men Is Finally Within Reach
|
A non-hormonal pill could soon expand men’s birth control options
|
sAC
Soluble Adenylyl Cyclase
Summary
This enzyme is necessary for the motility of sperm, as well as aspects of its ability to fertilize an egg.
The Science
Soluble adenylyl cyclase (sAC) is a regulatory cytosolic enzyme present in almost every cell. sAC is a source of cyclic adenosine 3’,5’ monophosphate (cAMP) – a second messenger that mediates cell growth and differentiation in organisms from bacteria to higher eukaryotes. In sperm, sAC activation by bicarbonate is necessary for motility and other aspects of capacitation. Infertile men have been identified with defects in sAC and minimal other side effects, indicating that transient inhibition of sAC could serve as a method of male contraception. Molecules that inhibit sAC have been developed and are undergoing further investigation. (Learn more here.)
Publications
Garcia TX, Matzuk MM. Novel Genes of the Male Reproductive System: Potential Roles in Male Reproduction and as Non-hormonal Male Contraceptive Targets. Mol Reprod Dev. 2024 Oct;91(10):e70000. doi: 10.1002/mrd.70000. PMID: 39422082.
Balbach, M., Rossetti, T., Ferreira, J. et al. On-demand male contraception via acute inhibition of soluble adenylyl cyclase. Nat Commun 14, 637 (2023). https://doi.org/10.1038/s41467-023-36119-6
Ayoub S, Rivera Sanchez NDR, Fischoeder J, Balbach M, Levin LR, Buck J, Ritagliati C. Cyclic AMP Rescue of Motility in Sperm Devoid of Soluble Adenylyl Cyclase. Int J Mol Sci. 2025 Feb 11;26(4):1489. doi: 10.3390/ijms26041489. PMID: 40003956; PMCID: PMC11855772.
Buffone MG, Wertheimer EV, Visconti PE, Krapf D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2610-20. doi: 10.1016/j.bbadis.2014.07.013. Epub 2014 Jul 24. PMID: 25066614; PMCID: PMC4262597.
Rossetti, T (Apr 2021). "Bicarbonate, carbon dioxide and pH sensing via mammalian bicarbonate-regulated soluble adenylyl cyclase". Interface Focus. 11 (2). doi:10.1098/rsfs.2020.0034. PMID 33633833.
Vacquier VD, Loza-Huerta A, García-Rincón J, Darszon A, Beltrán C. Soluble adenylyl cyclase of sea urchin spermatozoa. Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2621-8. doi: 10.1016/j.bbadis.2014.07.011. Epub 2014 Jul 23. PMID: 25064590; PMCID: PMC4262560.
This enzyme is necessary for the motility of sperm, as well as aspects of its ability to fertilize an egg.
The Science
Soluble adenylyl cyclase (sAC) is a regulatory cytosolic enzyme present in almost every cell. sAC is a source of cyclic adenosine 3’,5’ monophosphate (cAMP) – a second messenger that mediates cell growth and differentiation in organisms from bacteria to higher eukaryotes. In sperm, sAC activation by bicarbonate is necessary for motility and other aspects of capacitation. Infertile men have been identified with defects in sAC and minimal other side effects, indicating that transient inhibition of sAC could serve as a method of male contraception. Molecules that inhibit sAC have been developed and are undergoing further investigation. (Learn more here.)
Publications
Garcia TX, Matzuk MM. Novel Genes of the Male Reproductive System: Potential Roles in Male Reproduction and as Non-hormonal Male Contraceptive Targets. Mol Reprod Dev. 2024 Oct;91(10):e70000. doi: 10.1002/mrd.70000. PMID: 39422082.
Balbach, M., Rossetti, T., Ferreira, J. et al. On-demand male contraception via acute inhibition of soluble adenylyl cyclase. Nat Commun 14, 637 (2023). https://doi.org/10.1038/s41467-023-36119-6
Ayoub S, Rivera Sanchez NDR, Fischoeder J, Balbach M, Levin LR, Buck J, Ritagliati C. Cyclic AMP Rescue of Motility in Sperm Devoid of Soluble Adenylyl Cyclase. Int J Mol Sci. 2025 Feb 11;26(4):1489. doi: 10.3390/ijms26041489. PMID: 40003956; PMCID: PMC11855772.
Buffone MG, Wertheimer EV, Visconti PE, Krapf D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2610-20. doi: 10.1016/j.bbadis.2014.07.013. Epub 2014 Jul 24. PMID: 25066614; PMCID: PMC4262597.
Rossetti, T (Apr 2021). "Bicarbonate, carbon dioxide and pH sensing via mammalian bicarbonate-regulated soluble adenylyl cyclase". Interface Focus. 11 (2). doi:10.1098/rsfs.2020.0034. PMID 33633833.
Vacquier VD, Loza-Huerta A, García-Rincón J, Darszon A, Beltrán C. Soluble adenylyl cyclase of sea urchin spermatozoa. Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2621-8. doi: 10.1016/j.bbadis.2014.07.011. Epub 2014 Jul 23. PMID: 25064590; PMCID: PMC4262560.
|
MCI Grantee & Fellow Conversations: Mario Buffone
|
MCI Grantee & Fellow Conversations: Melanie Balbach
|
“On demand contraception via acute inhibition of soluble adenylyl cyclase” by Dra. Melanie Balbach
|
Male Birth Control Pills
|
Adenylyl Cyclase and cAMP
|
SLO3
SLO3 Potassium Channel
Summary
This is an ion channel necessary for male fertility; preventing its proper functioning could lead to infertility in men.
The Science
Slo3, or KCNU1, is a pH-sensitive and weakly voltage-sensitive potassium channel that is essential for male fertility in mice and whose expression is regarded as sperm-specific. Gene knock-out studies have unequivocally demonstrated the importance of the calcium and potassium conductances in sperm for fertility. In both species, the calcium current is carried by the highly complex cation channel of sperm (CatSper). In mouse sperm, the potassium current has been conclusively shown to be carried by a channel consisting of the pore forming subunit SLO3 and auxiliary subunit leucine-rich repeat-containing 52 (LRRC52). However, in human sperm it is controversial whether the pore forming subunit of the channel is composed of SLO3 and/or SLO1.
Publications
de Prelle B, Lybaert P and Gall D (2022) A Minimal Model Shows that a Positive Feedback Loop Between sNHE and SLO3 can Control Mouse Sperm Capacitation. Front. Cell Dev. Biol. 10:835594. doi: 10.3389/fcell.2022.835594
Ji N, Wang X, Zeng X, Kang H. Pharmacological inhibition of KSper impairs flagellar pH homeostasis of human spermatozoa. Andrology. 2024 Nov 5. doi: 10.1111/andr.13796. Epub ahead of print. PMID: 39498893.
Novero AG, Torres Rodríguez P, De la Vega Beltrán JL, Schiavi-Ehrenhaus LJ, Luque GM, Carruba M, Stival C, Gentile I, Ritagliati C, Santi CM, Nishigaki T, Krapf D, Buffone MG, Darszon A, Treviño CL, Krapf D. The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm. J Biol Chem. 2024 Dec;300(12):107932. doi: 10.1016/j.jbc.2024.107932. Epub 2024 Oct 28. PMID: 39476963; PMCID: PMC11629550.
Liu R, Yan Z, Fan Y, Qu R, Chen B, Li B, Wu L, Wu H, Mu J, Zhao L, Wang W, Dong J, Zeng Y, Li Q, Wang L, Sang Q, Zhang Z, Kuang Y. Bi-allelic variants in KCNU1 cause impaired acrosome reactions and male infertility. Hum Reprod. 2022 Jun 30;37(7):1394-1405. doi: 10.1093/humrep/deac102. PMID: 35551387.
Chávez JC, Vicens A, Wrighton DC, Andrade-López K, Beltrán C, Gutiérrez RM, Lippiat JD, Treviño CL. A cytoplasmic Slo3 isoform is expressed in somatic tissues. Mol Biol Rep. 2019 Oct;46(5):5561-5567. doi: 10.1007/s11033-019-04943-z. Epub 2019 Jul 3. PMID: 31270758.
Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011 Oct;91(4):1305-55. doi: 10.1152/physrev.00028.2010. PMID: 22013213.
Brown SG, Publicover SJ, Barratt CLR, Martins da Silva SJ. Human sperm ion channel (dys)function: implications for fertilization. Hum Reprod Update. 2019 Nov 5;25(6):758-776. doi: 10.1093/humupd/dmz032. PMID: 31665287; PMCID: PMC6847974.
This is an ion channel necessary for male fertility; preventing its proper functioning could lead to infertility in men.
The Science
Slo3, or KCNU1, is a pH-sensitive and weakly voltage-sensitive potassium channel that is essential for male fertility in mice and whose expression is regarded as sperm-specific. Gene knock-out studies have unequivocally demonstrated the importance of the calcium and potassium conductances in sperm for fertility. In both species, the calcium current is carried by the highly complex cation channel of sperm (CatSper). In mouse sperm, the potassium current has been conclusively shown to be carried by a channel consisting of the pore forming subunit SLO3 and auxiliary subunit leucine-rich repeat-containing 52 (LRRC52). However, in human sperm it is controversial whether the pore forming subunit of the channel is composed of SLO3 and/or SLO1.
Publications
de Prelle B, Lybaert P and Gall D (2022) A Minimal Model Shows that a Positive Feedback Loop Between sNHE and SLO3 can Control Mouse Sperm Capacitation. Front. Cell Dev. Biol. 10:835594. doi: 10.3389/fcell.2022.835594
Ji N, Wang X, Zeng X, Kang H. Pharmacological inhibition of KSper impairs flagellar pH homeostasis of human spermatozoa. Andrology. 2024 Nov 5. doi: 10.1111/andr.13796. Epub ahead of print. PMID: 39498893.
Novero AG, Torres Rodríguez P, De la Vega Beltrán JL, Schiavi-Ehrenhaus LJ, Luque GM, Carruba M, Stival C, Gentile I, Ritagliati C, Santi CM, Nishigaki T, Krapf D, Buffone MG, Darszon A, Treviño CL, Krapf D. The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm. J Biol Chem. 2024 Dec;300(12):107932. doi: 10.1016/j.jbc.2024.107932. Epub 2024 Oct 28. PMID: 39476963; PMCID: PMC11629550.
Liu R, Yan Z, Fan Y, Qu R, Chen B, Li B, Wu L, Wu H, Mu J, Zhao L, Wang W, Dong J, Zeng Y, Li Q, Wang L, Sang Q, Zhang Z, Kuang Y. Bi-allelic variants in KCNU1 cause impaired acrosome reactions and male infertility. Hum Reprod. 2022 Jun 30;37(7):1394-1405. doi: 10.1093/humrep/deac102. PMID: 35551387.
Chávez JC, Vicens A, Wrighton DC, Andrade-López K, Beltrán C, Gutiérrez RM, Lippiat JD, Treviño CL. A cytoplasmic Slo3 isoform is expressed in somatic tissues. Mol Biol Rep. 2019 Oct;46(5):5561-5567. doi: 10.1007/s11033-019-04943-z. Epub 2019 Jul 3. PMID: 31270758.
Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011 Oct;91(4):1305-55. doi: 10.1152/physrev.00028.2010. PMID: 22013213.
Brown SG, Publicover SJ, Barratt CLR, Martins da Silva SJ. Human sperm ion channel (dys)function: implications for fertilization. Hum Reprod Update. 2019 Nov 5;25(6):758-776. doi: 10.1093/humupd/dmz032. PMID: 31665287; PMCID: PMC6847974.
|
MCI Grantee & Fellow Conversations: Max Lyon
|
Introduction to Ion Channels: The role and function of potassium channels
|
Tamsulosin
Tamsulosin
Summary
Tamsulosin is a drug used in men to treat the symptoms of an enlarged prostate, marketed under the name Flomax. A common side effect of tamsulosin is ejaculatory dysfunction.
The Science
Tamsulosin is a selective antagonist at alpha-1A and alpha-1B-adrenoceptors in the prostate, prostatic capsule, prostatic urethra, and bladder neck. Mice knockout models of the alpha-1A and other adrenoreceptors created mice with notable reduction in fertility, and a combination knockout of the alpha-1A adrenoceptor alongside the P2X1-purinoreceptor resulted in 100% infertility without effects on sexual behavior or function. Combination therapies targeting both receptors are possible, resulting in a contraceptive that prevents ejaculation and the presence of sperm in any ejaculatory fluids. (Learn more here.)
Publications
Olayinka ET, Adewole KE. Ameliorative effect of morin on dutasteride-tamsulosin-induced testicular oxidative stress in rat. J Complement Integr Med. 2020 Dec 23;18(2):327-337. doi: 10.1515/jcim-2019-0160. PMID: 34187124.
Stadler B, Nowell CJ, Whittaker MR, Arnhold S, Pilatz A, Wagenlehner FM, Exintaris B, Middendorff R. Physiological and pharmacological impact of oxytocin on epididymal propulsion during the ejaculatory process in rodents and men. FASEB J. 2021 Jun;35(6):e21639. doi: 10.1096/fj.202100435R. PMID: 34041782.
Drobnis EZ, Nangia AK. Cardiovascular/Pulmonary Medications and Male Reproduction. Adv Exp Med Biol. 2017;1034:103-130. doi: 10.1007/978-3-319-69535-8_9. PMID: 29256129.
Dunn, P. M. “Fertility: Purinergic Receptors and the Male Contraceptive Pill.” Current Biology: CB 10, no. 8 (April 20, 2000): R305-307. https://doi.org/10.1016/s0960-9822(00)00436-x.
Sanbe, A., Y. Tanaka, Y. Fujiwara, H. Tsumura, J. Yamauchi, S. Cotecchia, K. Koike, G. Tsujimoto, and A. Tanoue. “Alpha1-Adrenoceptors Are Required for Normal Male Sexual Function.” British Journal of Pharmacology 152, no. 3 (October 2007): 332–40. https://doi.org/10.1038/sj.bjp.0707366.
White, Carl W., Yan-Ting Choong, Jennifer L. Short, Betty Exintaris, Daniel T. Malone, Andrew M. Allen, Richard J. Evans, and Sabatino Ventura. “Male Contraception via Simultaneous Knockout of Α1A-Adrenoceptors and P2X1-Purinoceptors in Mice.” Proceedings of the National Academy of Sciences of the United States of America 110, no. 51 (December 17, 2013): 20825–30. https://doi.org/10.1073/pnas.1318624110.
Tamsulosin is a drug used in men to treat the symptoms of an enlarged prostate, marketed under the name Flomax. A common side effect of tamsulosin is ejaculatory dysfunction.
The Science
Tamsulosin is a selective antagonist at alpha-1A and alpha-1B-adrenoceptors in the prostate, prostatic capsule, prostatic urethra, and bladder neck. Mice knockout models of the alpha-1A and other adrenoreceptors created mice with notable reduction in fertility, and a combination knockout of the alpha-1A adrenoceptor alongside the P2X1-purinoreceptor resulted in 100% infertility without effects on sexual behavior or function. Combination therapies targeting both receptors are possible, resulting in a contraceptive that prevents ejaculation and the presence of sperm in any ejaculatory fluids. (Learn more here.)
Publications
Olayinka ET, Adewole KE. Ameliorative effect of morin on dutasteride-tamsulosin-induced testicular oxidative stress in rat. J Complement Integr Med. 2020 Dec 23;18(2):327-337. doi: 10.1515/jcim-2019-0160. PMID: 34187124.
Stadler B, Nowell CJ, Whittaker MR, Arnhold S, Pilatz A, Wagenlehner FM, Exintaris B, Middendorff R. Physiological and pharmacological impact of oxytocin on epididymal propulsion during the ejaculatory process in rodents and men. FASEB J. 2021 Jun;35(6):e21639. doi: 10.1096/fj.202100435R. PMID: 34041782.
Drobnis EZ, Nangia AK. Cardiovascular/Pulmonary Medications and Male Reproduction. Adv Exp Med Biol. 2017;1034:103-130. doi: 10.1007/978-3-319-69535-8_9. PMID: 29256129.
Dunn, P. M. “Fertility: Purinergic Receptors and the Male Contraceptive Pill.” Current Biology: CB 10, no. 8 (April 20, 2000): R305-307. https://doi.org/10.1016/s0960-9822(00)00436-x.
Sanbe, A., Y. Tanaka, Y. Fujiwara, H. Tsumura, J. Yamauchi, S. Cotecchia, K. Koike, G. Tsujimoto, and A. Tanoue. “Alpha1-Adrenoceptors Are Required for Normal Male Sexual Function.” British Journal of Pharmacology 152, no. 3 (October 2007): 332–40. https://doi.org/10.1038/sj.bjp.0707366.
White, Carl W., Yan-Ting Choong, Jennifer L. Short, Betty Exintaris, Daniel T. Malone, Andrew M. Allen, Richard J. Evans, and Sabatino Ventura. “Male Contraception via Simultaneous Knockout of Α1A-Adrenoceptors and P2X1-Purinoceptors in Mice.” Proceedings of the National Academy of Sciences of the United States of America 110, no. 51 (December 17, 2013): 20825–30. https://doi.org/10.1073/pnas.1318624110.
|
MCI Grantee & Fellow Conversations: Sab Ventura
|
Tamsulosin and Finasteride SIDE EFFECTS that will SHOCK YOU! | Are they reversible?!
|
Triptonide
Tripterygium Wilfordii
Summary
Triptonide is a small molecule derived from a vine used in traditional Chinese medicine, and a single daily oral dose of it has shown to induce deformed sperm with minimal or no forward motility and consequently male infertility.
The Science
Tripterygium wilfordii, or léi gōng téng (Mandarin), sometimes called thunder god vine but more properly translated thunder duke vine, is a vine used in traditional Chinese medicine. Tripterygium wilfordii has been promoted for use in rheumatoid arthritis and psoriasis; however, due to safety concerns this use is not recommended. Evidence is insufficient to deem the plant effective as a method of birth control for humans, however a 2021 study published in Nature found that triptonide, a molecule isolated and synthesized, was an effective male birth control in mice and non-human primates. Triptonide affects the maturation of sperm, causing large defects in morphology that make sperm unable to swim or fertilize an egg. (Learn more here.)
Publications
Koilpillai JN, Nunan E, Butler L, Pinaffi F, Butcher JT. Reversible Contraception in Males: An Obtainable Target? Biology (Basel). 2024 Apr 25;13(5):291. doi: 10.3390/biology13050291. PMID: 38785772; PMCID: PMC11117788.
Song J, He GN, Dai L. A comprehensive review on celastrol, triptolide and triptonide: Insights on their pharmacological activity, toxicity, combination therapy, new dosage form and novel drug delivery routes. Biomed Pharmacother. 2023 Jun;162:114705. doi: 10.1016/j.biopha.2023.114705. Epub 2023 Apr 14. PMID: 37062220.
Shunnarah A, Tumlinson R, Calderón AI. Natural Products with Potential for Nonhormonal Male Contraception. J Nat Prod. 2021 Oct 22;84(10):2762-2774. doi: 10.1021/acs.jnatprod.1c00565. Epub 2021 Oct 11. PMID: 34633803.
Goldberg E. A plant root extract, triptonide, is a reversible male contraceptive in mice and monkeys. Biol Reprod. 2021 Jun 4;104(6):1187-1188. doi: 10.1093/biolre/ioab057. PMID: 33758919; PMCID: PMC8599777.
Chang, Z., Qin, W., Zheng, H. et al. Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat Commun 12, 1253 (2021). https://doi.org/10.1038/s41467-021-21517-5
Zheng, Huili, Clifford J. Stratton, Kazuto Morozumi, Jingling Jin, Ryuzo Yanagimachi, and Wei Yan. “Lack of Spem1 Causes Aberrant Cytoplasm Removal, Sperm Deformation, and Male Infertility.” Proceedings of the National Academy of Sciences of the United States of America 104, no. 16 (April 17, 2007): 6852–57. https://doi.org/10.1073/pnas.0701669104.
Yan, Wei. “Male Infertility Caused by Spermiogenic Defects: Lessons from Gene Knockouts.” Molecular and Cellular Endocrinology 306, no. 1–2 (July 10, 2009): 24–32. https://doi.org/10.1016/j.mce.2009.03.003.
Triptonide is a small molecule derived from a vine used in traditional Chinese medicine, and a single daily oral dose of it has shown to induce deformed sperm with minimal or no forward motility and consequently male infertility.
The Science
Tripterygium wilfordii, or léi gōng téng (Mandarin), sometimes called thunder god vine but more properly translated thunder duke vine, is a vine used in traditional Chinese medicine. Tripterygium wilfordii has been promoted for use in rheumatoid arthritis and psoriasis; however, due to safety concerns this use is not recommended. Evidence is insufficient to deem the plant effective as a method of birth control for humans, however a 2021 study published in Nature found that triptonide, a molecule isolated and synthesized, was an effective male birth control in mice and non-human primates. Triptonide affects the maturation of sperm, causing large defects in morphology that make sperm unable to swim or fertilize an egg. (Learn more here.)
Publications
Koilpillai JN, Nunan E, Butler L, Pinaffi F, Butcher JT. Reversible Contraception in Males: An Obtainable Target? Biology (Basel). 2024 Apr 25;13(5):291. doi: 10.3390/biology13050291. PMID: 38785772; PMCID: PMC11117788.
Song J, He GN, Dai L. A comprehensive review on celastrol, triptolide and triptonide: Insights on their pharmacological activity, toxicity, combination therapy, new dosage form and novel drug delivery routes. Biomed Pharmacother. 2023 Jun;162:114705. doi: 10.1016/j.biopha.2023.114705. Epub 2023 Apr 14. PMID: 37062220.
Shunnarah A, Tumlinson R, Calderón AI. Natural Products with Potential for Nonhormonal Male Contraception. J Nat Prod. 2021 Oct 22;84(10):2762-2774. doi: 10.1021/acs.jnatprod.1c00565. Epub 2021 Oct 11. PMID: 34633803.
Goldberg E. A plant root extract, triptonide, is a reversible male contraceptive in mice and monkeys. Biol Reprod. 2021 Jun 4;104(6):1187-1188. doi: 10.1093/biolre/ioab057. PMID: 33758919; PMCID: PMC8599777.
Chang, Z., Qin, W., Zheng, H. et al. Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat Commun 12, 1253 (2021). https://doi.org/10.1038/s41467-021-21517-5
Zheng, Huili, Clifford J. Stratton, Kazuto Morozumi, Jingling Jin, Ryuzo Yanagimachi, and Wei Yan. “Lack of Spem1 Causes Aberrant Cytoplasm Removal, Sperm Deformation, and Male Infertility.” Proceedings of the National Academy of Sciences of the United States of America 104, no. 16 (April 17, 2007): 6852–57. https://doi.org/10.1073/pnas.0701669104.
Yan, Wei. “Male Infertility Caused by Spermiogenic Defects: Lessons from Gene Knockouts.” Molecular and Cellular Endocrinology 306, no. 1–2 (July 10, 2009): 24–32. https://doi.org/10.1016/j.mce.2009.03.003.
MCI’s Lemonade Stand: “Triptonide as a Non-hormonal Male Contraceptive”
TSSKS
Testis-specific Serine Kinase Family (TSSK 1/2/3/6)
Summary
This family of proteins is found in the testis, and research has shown the inhibition of their function can lead to reversible infertility in male mammals.
The Science
Serine/Threonine Kinase receptors play a role in the regulation of cell proliferation, programmed cell death (apoptosis), cell differentiation, and embryonic development. Studies have shown that there is a subcellular localization of these kinases during spermatogenesis and in mature sperm. They are expressed in the testis, and research has shown inhibiting them can prevent spermatogenesis and/or fertilization. Due to this, they are considered an attractive target for reversible male contraception.
Publications
Nayyab S, Gervasi MG, Tourzani DA, Caraballo DA, Jha KN, Teves ME, Cui W, Georg GI, Visconti PE, Salicioni AM. TSSK3, a novel target for male contraception, is required for spermiogenesis. Mol Reprod Dev. 2021 Nov;88(11):718-730. doi: 10.1002/mrd.23539. Epub 2021 Oct 8. PMID: 34623009; PMCID: PMC8961454.
Ana M Salicioni, María G Gervasi, Julian Sosnik, Darya A Tourzani, Saman Nayyab, Diego A Caraballo, Pablo E Visconti, Testis-specific serine kinase protein family in male fertility and as targets for non-hormonal male contraception, Biology of Reproduction, Volume 103, Issue 2, August 2020, Pages 264–274
Ayoub R, Page ST, Swerdloff RS, Liu PY, Amory JK, Leung A, Hull L, Blithe D, Christy A, Chao JH, Bremner WJ, Wang C. Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive. Andrology. 2017 Mar;5(2):278-285.
Li Y, Sosnik J, Brassard L, Reese M, Spiridonov NA, Bates TC, Johnson GR, Anguita J, Visconti PE, Salicioni AM. Expression and localization of five members of the testis-specific serine kinase (Tssk) family in mouse and human sperm and testis. Mol Hum Reprod. 2011 Jan;17(1):42-56. doi: 10.1093/molehr/gaq071. Epub 2010 Aug 20. PMID: 20729278; PMCID: PMC3002843.
Kopf GS. Approaches to the identification of new nonhormonal targets for male contraception. Contraception. 2008 Oct;78(4 Suppl):S18-22.
This family of proteins is found in the testis, and research has shown the inhibition of their function can lead to reversible infertility in male mammals.
The Science
Serine/Threonine Kinase receptors play a role in the regulation of cell proliferation, programmed cell death (apoptosis), cell differentiation, and embryonic development. Studies have shown that there is a subcellular localization of these kinases during spermatogenesis and in mature sperm. They are expressed in the testis, and research has shown inhibiting them can prevent spermatogenesis and/or fertilization. Due to this, they are considered an attractive target for reversible male contraception.
Publications
Nayyab S, Gervasi MG, Tourzani DA, Caraballo DA, Jha KN, Teves ME, Cui W, Georg GI, Visconti PE, Salicioni AM. TSSK3, a novel target for male contraception, is required for spermiogenesis. Mol Reprod Dev. 2021 Nov;88(11):718-730. doi: 10.1002/mrd.23539. Epub 2021 Oct 8. PMID: 34623009; PMCID: PMC8961454.
Ana M Salicioni, María G Gervasi, Julian Sosnik, Darya A Tourzani, Saman Nayyab, Diego A Caraballo, Pablo E Visconti, Testis-specific serine kinase protein family in male fertility and as targets for non-hormonal male contraception, Biology of Reproduction, Volume 103, Issue 2, August 2020, Pages 264–274
Ayoub R, Page ST, Swerdloff RS, Liu PY, Amory JK, Leung A, Hull L, Blithe D, Christy A, Chao JH, Bremner WJ, Wang C. Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive. Andrology. 2017 Mar;5(2):278-285.
Li Y, Sosnik J, Brassard L, Reese M, Spiridonov NA, Bates TC, Johnson GR, Anguita J, Visconti PE, Salicioni AM. Expression and localization of five members of the testis-specific serine kinase (Tssk) family in mouse and human sperm and testis. Mol Hum Reprod. 2011 Jan;17(1):42-56. doi: 10.1093/molehr/gaq071. Epub 2010 Aug 20. PMID: 20729278; PMCID: PMC3002843.
Kopf GS. Approaches to the identification of new nonhormonal targets for male contraception. Contraception. 2008 Oct;78(4 Suppl):S18-22.
|
MCI Grantee & Fellow Conversations: Saman Nyyab
|
Interview with MCI Grantee Dr. Gunda Georg
|
Vasalgel
Vasalgel
Summary
Vasalgel is a high molecular weight polymer being developed in the U.S. as a contraceptive device for men. It is a similar formulation to RISUG whose technology was licensed to the U.S.-based Parsemus Foundation and is being developed as Vasalgel.
The Science
Vasalgel is a styrene maleic anhydride (SMA) dissolved in dimethyl sulfoxide (DMSO) and is formulated to adhere to strict regulatory standards. After injection into the vas deferens, the SMA acid forms a hydrogel that appears to be tissue adherent, fills the lumen and acts as a mechanical barrier to the passage of sperm. Vasalgel does not claim any pharmaceutical effect and is understood to work by occluding the vasa deferentia. (Learn more here.)
Publications
Buck KA, Stadick JL, Frazier ML. Preparing for sperm-targeted contraception: College students' perceptions and intentions related to non-hormonal intravas injectable gel. Public Health Nurs. 2020 Sep;37(5):639-646. doi: 10.1111/phn.12761. Epub 2020 Jul 5. PMID: 32627239.
Long JE, Lee MS, Blithe DL. Male Contraceptive Development: Update on Novel Hormonal and Nonhormonal Methods. Clin Chem. 2019 Jan;65(1):153-160. doi: 10.1373/clinchem.2018.295089. PMID: 30602479.
Khourdaji, I., Zillioux, J., Eisenfrats, K., Foley, D., & Smith, R. (2018). The future of male contraception: A fertile ground. Translational Andrology and Urology,7(S2).
Colagross-Schouten, A., Lemoy, M., Keesler, R. I., Lissner, E., & Vandevoort, C. A. (2017). The contraceptive efficacy of intravas injection of VasalgelTM for adult male rhesus monkeys. Basic and Clinical Andrology,27(1).
Waller D, Bolick D, Lissner E, Premanandan C, Gamerman G. Reversibility of Vasalgel™ male contraceptive in a rabbit model. Basic Clin Androl. 2017 Apr 5;27:8. doi: 10.1186/s12610-017-0051-1. PMID: 28417005; PMCID: PMC5381074.
Waller D, Bolick D, Lissner E, Premanandan C, Gamerman G. Azoospermia in rabbits following an intravas injection of Vasalgel ™. Basic Clin Androl. 2016;26:6. Published 2016 Mar 30. doi:10.1186/s12610-016-0033-8
Vasalgel is a high molecular weight polymer being developed in the U.S. as a contraceptive device for men. It is a similar formulation to RISUG whose technology was licensed to the U.S.-based Parsemus Foundation and is being developed as Vasalgel.
The Science
Vasalgel is a styrene maleic anhydride (SMA) dissolved in dimethyl sulfoxide (DMSO) and is formulated to adhere to strict regulatory standards. After injection into the vas deferens, the SMA acid forms a hydrogel that appears to be tissue adherent, fills the lumen and acts as a mechanical barrier to the passage of sperm. Vasalgel does not claim any pharmaceutical effect and is understood to work by occluding the vasa deferentia. (Learn more here.)
Publications
Buck KA, Stadick JL, Frazier ML. Preparing for sperm-targeted contraception: College students' perceptions and intentions related to non-hormonal intravas injectable gel. Public Health Nurs. 2020 Sep;37(5):639-646. doi: 10.1111/phn.12761. Epub 2020 Jul 5. PMID: 32627239.
Long JE, Lee MS, Blithe DL. Male Contraceptive Development: Update on Novel Hormonal and Nonhormonal Methods. Clin Chem. 2019 Jan;65(1):153-160. doi: 10.1373/clinchem.2018.295089. PMID: 30602479.
Khourdaji, I., Zillioux, J., Eisenfrats, K., Foley, D., & Smith, R. (2018). The future of male contraception: A fertile ground. Translational Andrology and Urology,7(S2).
Colagross-Schouten, A., Lemoy, M., Keesler, R. I., Lissner, E., & Vandevoort, C. A. (2017). The contraceptive efficacy of intravas injection of VasalgelTM for adult male rhesus monkeys. Basic and Clinical Andrology,27(1).
Waller D, Bolick D, Lissner E, Premanandan C, Gamerman G. Reversibility of Vasalgel™ male contraceptive in a rabbit model. Basic Clin Androl. 2017 Apr 5;27:8. doi: 10.1186/s12610-017-0051-1. PMID: 28417005; PMCID: PMC5381074.
Waller D, Bolick D, Lissner E, Premanandan C, Gamerman G. Azoospermia in rabbits following an intravas injection of Vasalgel ™. Basic Clin Androl. 2016;26:6. Published 2016 Mar 30. doi:10.1186/s12610-016-0033-8
L.R. Fox: Vasalgel, Male Birth Control | Whatever Podcast #2
|
Male contraceptive vasalgel passes animal tests
|
Male Contraception Conversations: Elaine Lissener
|
Join Our Research Community
Are you interested in working on one of these targets? We're eager to collaborate with researchers who are passionate about advancing male contraception. Contact us to discuss potential partnerships and funding opportunities.
Are you interested in working on one of these targets? We're eager to collaborate with researchers who are passionate about advancing male contraception. Contact us to discuss potential partnerships and funding opportunities.
Promising Targets for Male Contraception
Discover some of the most promising targets for non-hormonal, reversible male contraceptives that are currently being investigated by researchers worldwide. These targets have shown significant potential and are actively being explored by academic institutions and other organizations.
Explore the Pipeline: Learn more about these promising targets and the research being conducted to develop effective male birth control options.
Explore the Pipeline: Learn more about these promising targets and the research being conducted to develop effective male birth control options.
ABHD2
Abhydrolase Domain-containing Protein 2
Summary
This is an enzyme expressed in human spermatozoa that is a key controller of sperm hyperactivation, which is a necessary step in allowing sperm to fertilize an egg.
The Science
ABHD2 is an acylglycerol lipase enzyme that catalyzes endo-cannabinoids 1-arachidonoylglycerol (1AG) and 2-arachidonoylglycerol (2AG) only in the presence of progesterone (P4). AGs have been shown to act as inhibitors of CatSper, a sperm-specific calcium channel. Upon P4 stimulation, ABHD2 leads to the activation of CatSper, which results in sperm activation. Preventing ABHD2 activation of CatSper is one potential pathway towards a male contraceptive. Molecules have been identified that inhibit ABHD2-dependent CatSper activation, and are under investigation to improve their suitability as male contraceptives. (Learn more here.)
Publications
Baggelaar MP, den Dulk H, Florea BI, Fazio D, Bernabò N, Raspa M, Janssen APA, Scavizzi F, Barboni B, Overkleeft HS, Maccarrone M, van der Stelt M. ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction. ACS Chem Biol. 2019 Oct 18;14(10):2295-2304. doi: 10.1021/acschembio.9b00640. Epub 2019 Oct 1. Erratum in: ACS Chem Biol. 2019 Dec 20;14(12):2943. doi: 10.1021/acschembio.9b00824. PMID: 31525885; PMCID: PMC6878212.
"Preventing sperm's 'power kick' could be key to unisex contraceptive". Berkeley News. 2016-03-17. Retrieved 2017-09-13.
Mannowetz, Nadja; Miller, Melissa R.; Lishko, Polina V. (2017-05-30). "Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids". Proceedings of the National Academy of Sciences. 114 (22): 5743–5748. doi:10.1073/pnas.1700367114. ISSN 0027-8424. PMC 5465908. PMID 28507119.
Jin S, Zhao G, Li Z, Nishimoto Y, Isohama Y, Shen J, Ito T, Takeya M, Araki K, He P, Yamamura K (Mar 2009). "Age-related pulmonary emphysema in mice lacking alpha/beta hydrolase domain containing 2 gene". Biochemical and Biophysical Research Communications. 380 (2): 419–24.
This is an enzyme expressed in human spermatozoa that is a key controller of sperm hyperactivation, which is a necessary step in allowing sperm to fertilize an egg.
The Science
ABHD2 is an acylglycerol lipase enzyme that catalyzes endo-cannabinoids 1-arachidonoylglycerol (1AG) and 2-arachidonoylglycerol (2AG) only in the presence of progesterone (P4). AGs have been shown to act as inhibitors of CatSper, a sperm-specific calcium channel. Upon P4 stimulation, ABHD2 leads to the activation of CatSper, which results in sperm activation. Preventing ABHD2 activation of CatSper is one potential pathway towards a male contraceptive. Molecules have been identified that inhibit ABHD2-dependent CatSper activation, and are under investigation to improve their suitability as male contraceptives. (Learn more here.)
Publications
Baggelaar MP, den Dulk H, Florea BI, Fazio D, Bernabò N, Raspa M, Janssen APA, Scavizzi F, Barboni B, Overkleeft HS, Maccarrone M, van der Stelt M. ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction. ACS Chem Biol. 2019 Oct 18;14(10):2295-2304. doi: 10.1021/acschembio.9b00640. Epub 2019 Oct 1. Erratum in: ACS Chem Biol. 2019 Dec 20;14(12):2943. doi: 10.1021/acschembio.9b00824. PMID: 31525885; PMCID: PMC6878212.
"Preventing sperm's 'power kick' could be key to unisex contraceptive". Berkeley News. 2016-03-17. Retrieved 2017-09-13.
Mannowetz, Nadja; Miller, Melissa R.; Lishko, Polina V. (2017-05-30). "Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids". Proceedings of the National Academy of Sciences. 114 (22): 5743–5748. doi:10.1073/pnas.1700367114. ISSN 0027-8424. PMC 5465908. PMID 28507119.
Jin S, Zhao G, Li Z, Nishimoto Y, Isohama Y, Shen J, Ito T, Takeya M, Araki K, He P, Yamamura K (Mar 2009). "Age-related pulmonary emphysema in mice lacking alpha/beta hydrolase domain containing 2 gene". Biochemical and Biophysical Research Communications. 380 (2): 419–24.
Folk contraceptive may lead to ‘molecular condoms’
ALDH1A1
Aldehyde Dehydrogenase 1 Family, Member A1
Summary
Blocking this enzyme could prevent the male body from producing sperm.
The Science
Also known as ALDH1A1 or retinaldehyde dehydrogenase 1 (RALDH1), this is an enzyme that in humans is encoded by the ALDH1A1 gene. ALDH1A2 is an enzyme involved in testicular retinoic acid biosynthesis that, if blocked, can arrest spermatogenesis. Small molecule inhibitors of ALDH1A2 are being developed as long-term oral, reversible male contraceptives, based on earlier work on bisdichloroacetyldiamines (BDADs).
Publications
Paik J, Snyder JM, Kim A, Haenisch M, Fogassy K, Amory JK. Kinetics of the inhibition and recovery of spermatogenesis induced by treatment with WIN 18,446, a male contraceptive, in mice. Andrology. 2024 Nov 20. doi: 10.1111/andr.13807. Epub ahead of print. PMID: 39564943.
Pereira F, Rosenmann E, Nylen E, Kaufman M, Pinsky L, Wrogemann K (March 1991). "The 56 kDa androgen binding protein is an aldehyde dehydrogenase". Biochemical and Biophysical Research Communications. 175 (3): 831–8. doi:10.1016/0006-291X(91)91640-X. PMID 1709013.
Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A (June 1985). "Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2". Proceedings of the National Academy of Sciences of the United States of America. 82 (11): 3771–5. Bibcode:1985PNAS...82.3771H. doi:10.1073/pnas.82.11.3771. PMC 397869. PMID 2987944.
Blocking this enzyme could prevent the male body from producing sperm.
The Science
Also known as ALDH1A1 or retinaldehyde dehydrogenase 1 (RALDH1), this is an enzyme that in humans is encoded by the ALDH1A1 gene. ALDH1A2 is an enzyme involved in testicular retinoic acid biosynthesis that, if blocked, can arrest spermatogenesis. Small molecule inhibitors of ALDH1A2 are being developed as long-term oral, reversible male contraceptives, based on earlier work on bisdichloroacetyldiamines (BDADs).
Publications
Paik J, Snyder JM, Kim A, Haenisch M, Fogassy K, Amory JK. Kinetics of the inhibition and recovery of spermatogenesis induced by treatment with WIN 18,446, a male contraceptive, in mice. Andrology. 2024 Nov 20. doi: 10.1111/andr.13807. Epub ahead of print. PMID: 39564943.
Pereira F, Rosenmann E, Nylen E, Kaufman M, Pinsky L, Wrogemann K (March 1991). "The 56 kDa androgen binding protein is an aldehyde dehydrogenase". Biochemical and Biophysical Research Communications. 175 (3): 831–8. doi:10.1016/0006-291X(91)91640-X. PMID 1709013.
Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A (June 1985). "Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2". Proceedings of the National Academy of Sciences of the United States of America. 82 (11): 3771–5. Bibcode:1985PNAS...82.3771H. doi:10.1073/pnas.82.11.3771. PMC 397869. PMID 2987944.
Aldehyde Dehydrogenase 1
ANT4
Adenine Nucleotide Translocase 4
Summary
This is the most abundant protein in male germ cell mitochondria, and reducing its presence could make men infertile.
The Science
This protein facilitates ADP (adenosine diphosphate) / ATP (adenosine triphosphate) exchange across the mitochondrial inner membrane and is exclusively expressed in male germ cells. Ant4 expression is particularly high during male meiosis and is essential for progression of male meiosis. Targeted depletion of Ant4 in mice has resulted in male infertility, and it has also been hypothesized that premature activation of ANT4 could drain sperm of the energy required to reach and fertilize an egg. (Learn more here.)
Publications
Yang F, Yang X, Zhu H, Wang X, Liao X, Fu Y, Fu T, Chen X, Sysa A, Lyu J, Zhou H. The essential role of adenine nucleotide translocase 4 on male reproductive function in mice. Braz J Med Biol Res. 2024 May 20;57:e13590. doi: 10.1590/1414-431X2024e13590. PMID: 38808891; PMCID: PMC11136480.
Kunji ER, Aleksandrova A, King MS, Majd H, Ashton VL, Cerson E, Springett R, Kibalchenko M, Tavoulari S, Crichton PG, Ruprecht JJ (October 2016). "The transport mechanism of the mitochondrial ADP/ATP carrier". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. Channels and transporters in cell metabolism. 1863 (10): 2379–93.
Leung WY, Hamazaki T, Ostrov DA, Terada N. Identification of adenine nucleotide translocase 4 inhibitors by molecular docking. J Mol Graph Model. 2013 Sep;45:173-9. doi: 10.1016/j.jmgm.2013.08.016. Epub 2013 Sep 4. PMID: 24056384; PMCID: PMC3821932.
Klingenberg M (October 2008). "The ADP and ATP transport in mitochondria and its carrier". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1778 (10): 1978–2021. doi:10.1016/j.bbamem.2008.04.011. PMID 18510943.
This is the most abundant protein in male germ cell mitochondria, and reducing its presence could make men infertile.
The Science
This protein facilitates ADP (adenosine diphosphate) / ATP (adenosine triphosphate) exchange across the mitochondrial inner membrane and is exclusively expressed in male germ cells. Ant4 expression is particularly high during male meiosis and is essential for progression of male meiosis. Targeted depletion of Ant4 in mice has resulted in male infertility, and it has also been hypothesized that premature activation of ANT4 could drain sperm of the energy required to reach and fertilize an egg. (Learn more here.)
Publications
Yang F, Yang X, Zhu H, Wang X, Liao X, Fu Y, Fu T, Chen X, Sysa A, Lyu J, Zhou H. The essential role of adenine nucleotide translocase 4 on male reproductive function in mice. Braz J Med Biol Res. 2024 May 20;57:e13590. doi: 10.1590/1414-431X2024e13590. PMID: 38808891; PMCID: PMC11136480.
Kunji ER, Aleksandrova A, King MS, Majd H, Ashton VL, Cerson E, Springett R, Kibalchenko M, Tavoulari S, Crichton PG, Ruprecht JJ (October 2016). "The transport mechanism of the mitochondrial ADP/ATP carrier". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. Channels and transporters in cell metabolism. 1863 (10): 2379–93.
Leung WY, Hamazaki T, Ostrov DA, Terada N. Identification of adenine nucleotide translocase 4 inhibitors by molecular docking. J Mol Graph Model. 2013 Sep;45:173-9. doi: 10.1016/j.jmgm.2013.08.016. Epub 2013 Sep 4. PMID: 24056384; PMCID: PMC3821932.
Klingenberg M (October 2008). "The ADP and ATP transport in mitochondria and its carrier". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1778 (10): 1978–2021. doi:10.1016/j.bbamem.2008.04.011. PMID 18510943.
|
The Adenine Nucleotide Transporter
|
ATP-ADP Translocase
|
BRDT
Bromodomain Testis-specific Gene
Summary
This is a protein coding gene, and inhibiting its function could cause a reversible reduction of sperm production and quality.
The Science
BRDT is a transcriptional regulator that belongs to the bromodomain and extra-terminal (BET) subfamily of epigenetic reader proteins. BRDT is predominantly expressed in the testis and is also observed in the mouse pituitary gland. BRDT is involved in chromatin organization and transcriptional regulation during spermatogenesis. It is also shown to play a role in scheduling male puberty through the regulation of the pituitary-gonadal axis. BRDT-null male mice are infertile and demonstrate severe reproductive phenotypes. The small molecule, JQ1, is a lead compound in targeting BRDT. (Learn more here.)
Publications
Wu S, Li X, Shang L, Wu L, Li T, Li P, Ji Z, Hou J, Yin M, Xu W. The novel BRDT inhibitor NHWD870 shows potential as a male contraceptive in mice. Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(12):1789-1800. doi: 10.3724/abbs.2022135. PMID: 36239350; PMCID: PMC10157631.
Kohandani F, Jazireian P, Favaedi R, Sadighi Gilani MA, Moshtaghioun SM, Shahhoseini M. Epigenetic Dysregulation of BRDT Gene in Testis Tissues of Infertile Men: Case-Control Study. Cell J. 2022 Feb;24(2):99-102. doi: 10.22074/cellj.2022.7724. PMID: 35279966; PMCID: PMC8918272.
Jones MH, Numata M, Shimane M (Jan 1998). "Identification and characterization of BRDT: A testis-specific gene related to the bromodomain genes RING3 and Drosophila fsh". Genomics. 45 (3): 529–34. doi:10.1006/geno.1997.5000. PMID 9367677.
Gaucher J, Boussouar F, Montellier E, Curtet S, Buchou T, Bertrand S, Hery P, Jounier S, Depaux A, Vitte AL, Guardiola P, Pernet K, Debernardi A, Lopez F, Holota H, Imbert J, Wolgemuth DJ, Gérard M, Rousseaux S, Khochbin S (2012). "Bromodomain-dependent stage-specific male genome programming by Brdt". EMBO J. 31 (19): 3809–20. doi:10.1038/emboj.2012.233. PMC 3463845. PMID 22922464.
Skarnes, W. C.; Rosen, B.; West, A. P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A. O.; Thomas, M.; Harrow, J.; Cox, T.; Jackson, D.; Severin, J.; Biggs, P.; Fu, J.; Nefedov, M.; De Jong, P. J.; Stewart, A. F.; Bradley, A. (2011). "A conditional knockout resource for the genome-wide study of mouse gene function". Nature. 474 (7351): 337–342. doi:10.1038/nature10163. PMC 3572410. PMID 21677750.
This is a protein coding gene, and inhibiting its function could cause a reversible reduction of sperm production and quality.
The Science
BRDT is a transcriptional regulator that belongs to the bromodomain and extra-terminal (BET) subfamily of epigenetic reader proteins. BRDT is predominantly expressed in the testis and is also observed in the mouse pituitary gland. BRDT is involved in chromatin organization and transcriptional regulation during spermatogenesis. It is also shown to play a role in scheduling male puberty through the regulation of the pituitary-gonadal axis. BRDT-null male mice are infertile and demonstrate severe reproductive phenotypes. The small molecule, JQ1, is a lead compound in targeting BRDT. (Learn more here.)
Publications
Wu S, Li X, Shang L, Wu L, Li T, Li P, Ji Z, Hou J, Yin M, Xu W. The novel BRDT inhibitor NHWD870 shows potential as a male contraceptive in mice. Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(12):1789-1800. doi: 10.3724/abbs.2022135. PMID: 36239350; PMCID: PMC10157631.
Kohandani F, Jazireian P, Favaedi R, Sadighi Gilani MA, Moshtaghioun SM, Shahhoseini M. Epigenetic Dysregulation of BRDT Gene in Testis Tissues of Infertile Men: Case-Control Study. Cell J. 2022 Feb;24(2):99-102. doi: 10.22074/cellj.2022.7724. PMID: 35279966; PMCID: PMC8918272.
Jones MH, Numata M, Shimane M (Jan 1998). "Identification and characterization of BRDT: A testis-specific gene related to the bromodomain genes RING3 and Drosophila fsh". Genomics. 45 (3): 529–34. doi:10.1006/geno.1997.5000. PMID 9367677.
Gaucher J, Boussouar F, Montellier E, Curtet S, Buchou T, Bertrand S, Hery P, Jounier S, Depaux A, Vitte AL, Guardiola P, Pernet K, Debernardi A, Lopez F, Holota H, Imbert J, Wolgemuth DJ, Gérard M, Rousseaux S, Khochbin S (2012). "Bromodomain-dependent stage-specific male genome programming by Brdt". EMBO J. 31 (19): 3809–20. doi:10.1038/emboj.2012.233. PMC 3463845. PMID 22922464.
Skarnes, W. C.; Rosen, B.; West, A. P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A. O.; Thomas, M.; Harrow, J.; Cox, T.; Jackson, D.; Severin, J.; Biggs, P.; Fu, J.; Nefedov, M.; De Jong, P. J.; Stewart, A. F.; Bradley, A. (2011). "A conditional knockout resource for the genome-wide study of mouse gene function". Nature. 474 (7351): 337–342. doi:10.1038/nature10163. PMC 3572410. PMID 21677750.
PPP3CC / PPP3R2
Protein Phosphatase 3 Catalytic Subunit Gamma / Protein Phosphatase 3 Regulatory Subunit B, Beta
Summary
PPP3CC and PPP3R2 are genes found in the testis that are necessary for the development and function of sperm.
The Science
The testis expresses the somatic phosphatase calcineurin, composed of a catalytic subunit (i.e., PPP3CC) and a regulatory subunit (i.e., PPP3R2). PPP3CC and PPP3R2 have been found to be strongly expressed in the testis, and studies have demonstrated that male mice lacking Ppp3cc or Ppp3r2 genes are infertile, with reduced sperm motility owing to an inflexible midpiece. Molecules that affect sperm calcineurine have been identified that cause infertility in mice, however, fertility returned in mice a week after treatment was stopped, indicating the potential for these molecules to be investigated as pathway towards reversible male contraceptives.
Publications
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016 Nov 10;7(11):e2472. doi: 10.1038/cddis.2016.344. PMID: 27831554; PMCID: PMC5260884.
Miyata H, Oura S, Morohoshi A, Shimada K, Mashiko D, Oyama Y, Kaneda Y, Matsumura T, Abbasi F, Ikawa M. SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2106673118. doi: 10.1073/pnas.2106673118. PMID: 34446558; PMCID: PMC8536318.
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016 Nov 10;7(11):e2472.
Miyata H, Satouh Y, Mashiko D, Muto M, Nozawa K, Shiba K, Fujihara Y, Isotani A, Inaba K, Ikawa M. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015 Oct 23;350(6259):442-5.
Muramatsu T, Kincaid RL (Nov 1992). "Molecular cloning and chromosomal mapping of the human gene for the testis-specific catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A)". Biochem Biophys Res Commun. 188 (1): 265–71.
PPP3CC and PPP3R2 are genes found in the testis that are necessary for the development and function of sperm.
The Science
The testis expresses the somatic phosphatase calcineurin, composed of a catalytic subunit (i.e., PPP3CC) and a regulatory subunit (i.e., PPP3R2). PPP3CC and PPP3R2 have been found to be strongly expressed in the testis, and studies have demonstrated that male mice lacking Ppp3cc or Ppp3r2 genes are infertile, with reduced sperm motility owing to an inflexible midpiece. Molecules that affect sperm calcineurine have been identified that cause infertility in mice, however, fertility returned in mice a week after treatment was stopped, indicating the potential for these molecules to be investigated as pathway towards reversible male contraceptives.
Publications
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016 Nov 10;7(11):e2472. doi: 10.1038/cddis.2016.344. PMID: 27831554; PMCID: PMC5260884.
Miyata H, Oura S, Morohoshi A, Shimada K, Mashiko D, Oyama Y, Kaneda Y, Matsumura T, Abbasi F, Ikawa M. SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2106673118. doi: 10.1073/pnas.2106673118. PMID: 34446558; PMCID: PMC8536318.
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016 Nov 10;7(11):e2472.
Miyata H, Satouh Y, Mashiko D, Muto M, Nozawa K, Shiba K, Fujihara Y, Isotani A, Inaba K, Ikawa M. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015 Oct 23;350(6259):442-5.
Muramatsu T, Kincaid RL (Nov 1992). "Molecular cloning and chromosomal mapping of the human gene for the testis-specific catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A)". Biochem Biophys Res Commun. 188 (1): 265–71.
Join Our Research Community
Are you interested in working on one of these targets? We're eager to collaborate with researchers who are passionate about advancing male contraception. Contact us to discuss potential partnerships and funding opportunities.
Are you interested in working on one of these targets? We're eager to collaborate with researchers who are passionate about advancing male contraception. Contact us to discuss potential partnerships and funding opportunities.
Exploring Emerging Targets for Male Contraception
This section highlights additional targets that have been identified as promising candidates for male contraception but require further research. We've categorized these targets based on the specific aspect of male reproduction they aim to impact.
For more information on these categories, please visit our page "The Drug Development Pipeline":
For more information on these categories, please visit our page "The Drug Development Pipeline":
Spermatogenesis
CALR3 (Calreticulin 3)
Summary
This gene is found in the testis and may be necessary for sperm fertility.
The Science
CALR3 and calmegin (CLGN, see profile) are lectin chaperones localized in the endoplasmic reticulum and cooperate to mediate nascent glycoprotein folding and play critical roles in calcium homeostasis. CALR3-null mice are almost completely sterile despite normal mating behavior. CLGN and CALR3 have distinct but cooperative roles in the maturation of ADAM3, and the onset of CLGN and CALR3 expression was considered to be meiotic and post-meiotic, respectively. It is notable that CLGN expression preceded that of CALR3, mirroring the offset expression of ADAM1B/ADAM2 and ADAM3. (Learn more here.)
Publications
Verhagen, J.M., Veldman, J.H., van der Zwaag, P.A. et al. Lack of evidence for a causal role of CALR3 in monogenic cardiomyopathy. Eur J Hum Genet 26, 1603–1610 (2018). https://doi.org/10.1038/s41431-018-0208-1
Ikawa M, Tokuhiro K, Yamaguchi R, Benham AM, Tamura T, Wada I, Satouh Y, Inoue N, Okabe M. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem. 2011 Feb 18;286(7):5639-46. doi: 10.1074/jbc.M110.140152. Epub 2010 Dec 3. PMID: 21131354; PMCID: PMC3037677.
This gene is found in the testis and may be necessary for sperm fertility.
The Science
CALR3 and calmegin (CLGN, see profile) are lectin chaperones localized in the endoplasmic reticulum and cooperate to mediate nascent glycoprotein folding and play critical roles in calcium homeostasis. CALR3-null mice are almost completely sterile despite normal mating behavior. CLGN and CALR3 have distinct but cooperative roles in the maturation of ADAM3, and the onset of CLGN and CALR3 expression was considered to be meiotic and post-meiotic, respectively. It is notable that CLGN expression preceded that of CALR3, mirroring the offset expression of ADAM1B/ADAM2 and ADAM3. (Learn more here.)
Publications
Verhagen, J.M., Veldman, J.H., van der Zwaag, P.A. et al. Lack of evidence for a causal role of CALR3 in monogenic cardiomyopathy. Eur J Hum Genet 26, 1603–1610 (2018). https://doi.org/10.1038/s41431-018-0208-1
Ikawa M, Tokuhiro K, Yamaguchi R, Benham AM, Tamura T, Wada I, Satouh Y, Inoue N, Okabe M. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem. 2011 Feb 18;286(7):5639-46. doi: 10.1074/jbc.M110.140152. Epub 2010 Dec 3. PMID: 21131354; PMCID: PMC3037677.
CFAP61 (Cilia And Flagella Associated Protein 61)
Summary
Variants of CFAP61 are associated with multiple morphological abnormalities of sperm flagella and male infertility in both mice and humans.
The Science
CFAP61 is a conserved component of the calmodulin- and radial spoke-associated complex (CSC) of cilia, with evidence in both mouse and human models. Cfap61 knockout mice recapitulate the infertility phenotype of the human CFAP61 mutation, but without other symptoms usually observed in other ciliary disorders. During early stages of Cfap61-/- spermatid development, the assembly of radial spoke components is impaired. As spermiogenesis progresses, the axoneme in Cfap61-/- cells becomes unstable and scatters, and the distribution of intraflagellar transport proteins is disrupted.
Publications
Hu T, Meng L, Tan C, Luo C, He WB, Tu C, Zhang H, Du J, Nie H, Lu GX, Lin G, Tan YQ. Biallelic CFAP61 variants cause male infertility in humans and mice with severe oligoasthenoteratozoospermia. J Med Genet. 2022 Apr 6:jmedgenet-2021-108249. doi: 10.1136/jmedgenet-2021-108249. Epub ahead of print. PMID: 35387802.
Ma A, Zeb A, Ali I, Zhao D, Khan A, Zhang B, Zhou J, Khan R, Zhang H, Zhang Y, Khan I, Shah W, Ali H, Javed AR, Ma H, Shi Q. Biallelic Variants in CFAP61 Cause Multiple Morphological Abnormalities of the Flagella and Male Infertility. Front Cell Dev Biol. 2022 Jan 31;9:803818. doi: 10.3389/fcell.2021.803818. PMID: 35174165; PMCID: PMC8841411.
Liu S, Zhang J, Kherraf ZE, Sun S, Zhang X, Cazin C, Coutton C, Zouari R, Zhao S, Hu F, Fourati Ben Mustapha S, Arnoult C, Ray PF, Liu M. CFAP61 is required for sperm flagellum formation and male fertility in human and mouse. Development. 2021 Dec 1;148(23):dev199805. doi: 10.1242/dev.199805. Epub 2021 Dec 7. PMID: 34792097.
Variants of CFAP61 are associated with multiple morphological abnormalities of sperm flagella and male infertility in both mice and humans.
The Science
CFAP61 is a conserved component of the calmodulin- and radial spoke-associated complex (CSC) of cilia, with evidence in both mouse and human models. Cfap61 knockout mice recapitulate the infertility phenotype of the human CFAP61 mutation, but without other symptoms usually observed in other ciliary disorders. During early stages of Cfap61-/- spermatid development, the assembly of radial spoke components is impaired. As spermiogenesis progresses, the axoneme in Cfap61-/- cells becomes unstable and scatters, and the distribution of intraflagellar transport proteins is disrupted.
Publications
Hu T, Meng L, Tan C, Luo C, He WB, Tu C, Zhang H, Du J, Nie H, Lu GX, Lin G, Tan YQ. Biallelic CFAP61 variants cause male infertility in humans and mice with severe oligoasthenoteratozoospermia. J Med Genet. 2022 Apr 6:jmedgenet-2021-108249. doi: 10.1136/jmedgenet-2021-108249. Epub ahead of print. PMID: 35387802.
Ma A, Zeb A, Ali I, Zhao D, Khan A, Zhang B, Zhou J, Khan R, Zhang H, Zhang Y, Khan I, Shah W, Ali H, Javed AR, Ma H, Shi Q. Biallelic Variants in CFAP61 Cause Multiple Morphological Abnormalities of the Flagella and Male Infertility. Front Cell Dev Biol. 2022 Jan 31;9:803818. doi: 10.3389/fcell.2021.803818. PMID: 35174165; PMCID: PMC8841411.
Liu S, Zhang J, Kherraf ZE, Sun S, Zhang X, Cazin C, Coutton C, Zouari R, Zhao S, Hu F, Fourati Ben Mustapha S, Arnoult C, Ray PF, Liu M. CFAP61 is required for sperm flagellum formation and male fertility in human and mouse. Development. 2021 Dec 1;148(23):dev199805. doi: 10.1242/dev.199805. Epub 2021 Dec 7. PMID: 34792097.
CFAP65 (Cilia And Flagella Associated Protein 65)
Summary
This gene is required for acrosome biogenesis and mitochondrial sheath assembly.
The Science
Multiple morphological abnormalities of the sperm flagella (MMAF) is a rare autosomal recessive inherited disorder associated with male infertility. Cilia and flagella-associated protein 65 (CFAP65) is primarily expressed in the testis and variants have been associated with infertility in both humans and mice. Observations on human subjects suggested that CFAP65 is involved in sperm flagellum structure and assembly and that loss-of-function mutations could lead to male infertility in humans by causing the MMAF phenotype, and mutations of CFAP65 have mimicked this function in a mouse model.
Publications
Li W, Wu H, Li F, Tian S, Kherraf ZE, Zhang J, Ni X, Lv M, Liu C, Tan Q, Shen Y, Amiri-Yekta A, Cazin C, Zhang J, Liu W, Zheng Y, Cheng H, Wu Y, Wang J, Gao Y, Chen Y, Zha X, Jin L, Liu M, He X, Ray PF, Cao Y, Zhang F. Biallelic mutations in CFAP65 cause male infertility with multiple morphological abnormalities of the sperm flagella in humans and mice. J Med Genet. 2020 Feb;57(2):89-95. doi: 10.1136/jmedgenet-2019-106344. Epub 2019 Sep 9. PMID: 31501240.
Zhang X, Shen Y, Wang X, Yuan G, Zhang C, Yang Y. A novel homozygous CFAP65 mutation in humans causes male infertility with multiple morphological abnormalities of the sperm flagella. Clin Genet. 2019 Dec;96(6):541-548. doi: 10.1111/cge.13644. Epub 2019 Oct 3. PMID: 31571197.
Wang W, Tian S, Nie H, Tu C, Liu C, Li Y, Li D, Yang X, Meng L, Hu T, Zhang Q, Du J, Fan L, Lu G, Lin G, Zhang F, Tan YQ. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum Mol Genet. 2021 Nov 16;30(23):2240-2254. doi: 10.1093/hmg/ddab185. PMID: 34231842.
This gene is required for acrosome biogenesis and mitochondrial sheath assembly.
The Science
Multiple morphological abnormalities of the sperm flagella (MMAF) is a rare autosomal recessive inherited disorder associated with male infertility. Cilia and flagella-associated protein 65 (CFAP65) is primarily expressed in the testis and variants have been associated with infertility in both humans and mice. Observations on human subjects suggested that CFAP65 is involved in sperm flagellum structure and assembly and that loss-of-function mutations could lead to male infertility in humans by causing the MMAF phenotype, and mutations of CFAP65 have mimicked this function in a mouse model.
Publications
Li W, Wu H, Li F, Tian S, Kherraf ZE, Zhang J, Ni X, Lv M, Liu C, Tan Q, Shen Y, Amiri-Yekta A, Cazin C, Zhang J, Liu W, Zheng Y, Cheng H, Wu Y, Wang J, Gao Y, Chen Y, Zha X, Jin L, Liu M, He X, Ray PF, Cao Y, Zhang F. Biallelic mutations in CFAP65 cause male infertility with multiple morphological abnormalities of the sperm flagella in humans and mice. J Med Genet. 2020 Feb;57(2):89-95. doi: 10.1136/jmedgenet-2019-106344. Epub 2019 Sep 9. PMID: 31501240.
Zhang X, Shen Y, Wang X, Yuan G, Zhang C, Yang Y. A novel homozygous CFAP65 mutation in humans causes male infertility with multiple morphological abnormalities of the sperm flagella. Clin Genet. 2019 Dec;96(6):541-548. doi: 10.1111/cge.13644. Epub 2019 Oct 3. PMID: 31571197.
Wang W, Tian S, Nie H, Tu C, Liu C, Li Y, Li D, Yang X, Meng L, Hu T, Zhang Q, Du J, Fan L, Lu G, Lin G, Zhang F, Tan YQ. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum Mol Genet. 2021 Nov 16;30(23):2240-2254. doi: 10.1093/hmg/ddab185. PMID: 34231842.
FAM209 (Family with sequence similarity 209)
Summary
This gene is required for sperm acrosome biogenesis and fertility in mice.
The Science
Family with sequence similarity 209 (Fam209) is required for acrosome biogenesis in mouse sperm. FAM209 is a small transmembrane protein conserved among mammals. Loss of Fam209 results in fertility defects that are secondary to abnormalities in acrosome biogenesis during spermiogenesis, reminiscent of globozoospermia. Analysis of the FAM209 proteome identified DPY19L2, whose human orthologue is involved in the majority of globozoospermia cases. Mutations in human and mouse Dpy19l2 have been shown to cause globozoospermia, and FAM209 colocalizes with DPY19L2 at the inner nuclear membrane to maintain the developing acrosome.
Publications
Castaneda JM, Shimada K, Satouh Y, Yu Z, Devlin DJ, Ikawa M, Matzuk MM. FAM209 associates with DPY19L2, and is required for sperm acrosome biogenesis and fertility in mice. J Cell Sci. 2021 Nov 1;134(21):jcs259206. doi: 10.1242/jcs.259206. Epub 2021 Nov 1. PMID: 34471926; PMCID: PMC8627553.
This gene is required for sperm acrosome biogenesis and fertility in mice.
The Science
Family with sequence similarity 209 (Fam209) is required for acrosome biogenesis in mouse sperm. FAM209 is a small transmembrane protein conserved among mammals. Loss of Fam209 results in fertility defects that are secondary to abnormalities in acrosome biogenesis during spermiogenesis, reminiscent of globozoospermia. Analysis of the FAM209 proteome identified DPY19L2, whose human orthologue is involved in the majority of globozoospermia cases. Mutations in human and mouse Dpy19l2 have been shown to cause globozoospermia, and FAM209 colocalizes with DPY19L2 at the inner nuclear membrane to maintain the developing acrosome.
Publications
Castaneda JM, Shimada K, Satouh Y, Yu Z, Devlin DJ, Ikawa M, Matzuk MM. FAM209 associates with DPY19L2, and is required for sperm acrosome biogenesis and fertility in mice. J Cell Sci. 2021 Nov 1;134(21):jcs259206. doi: 10.1242/jcs.259206. Epub 2021 Nov 1. PMID: 34471926; PMCID: PMC8627553.
FAM71F1 (Family with sequence similarity 71, member F1)
Summary
This gene is required for sperm acrosome biogenesis and fertility in mice.
The Science
Family with sequence similarity 71, member F1 (FAM71F1) is a testis-enriched protein that contains a RAB2B-binding domain, a small GTPase involved in vesicle transport and membrane trafficking. Fam71f1 is essential for male fertility. In Fam71f1-mutant mice, the acrosome was abnormally expanded at the round spermatid stage, likely because of enhanced vesicle trafficking. Mass spectrometry analysis after immunoprecipitation indicated that, in testes, FAM71F1 binds not only RAB2B, but also RAB2A. Further study suggested that FAM71F1 binds to the GTP-bound active form of RAB2A/B, but not the inactive form. These results indicate that a complex of FAM71F1 and active RAB2A/B suppresses excessive vesicle trafficking during acrosome formation.
Publications
Morohoshi A, Miyata H, Oyama Y, Oura S, Noda T, Ikawa M. FAM71F1 binds to RAB2A and RAB2B and is essential for acrosome formation and male fertility in mice. Development. 2021 Nov 1;148(21):dev199644. doi: 10.1242/dev.199644. Epub 2021 Oct 29. PMID: 34714330; PMCID: PMC8602946.
Malcher A, Rozwadowska N, Stokowy T, Kolanowski T, Jedrzejczak P, Zietkowiak W, Kurpisz M. Potential biomarkers of nonobstructive azoospermia identified in microarray gene expression analysis. Fertil Steril. 2013 Dec;100(6):1686-94.e1-7. doi: 10.1016/j.fertnstert.2013.07.1999. Epub 2013 Sep 4. PMID: 24012201.
Kolmykov S, Vasiliev G, Osadchuk L, Kleschev M, Osadchuk A. Whole-Exome Sequencing Analysis of Human Semen Quality in Russian Multiethnic Population. Front Genet. 2021 Jun 11;12:662846. doi: 10.3389/fgene.2021.662846. PMID: 34178030; PMCID: PMC8232892.
This gene is required for sperm acrosome biogenesis and fertility in mice.
The Science
Family with sequence similarity 71, member F1 (FAM71F1) is a testis-enriched protein that contains a RAB2B-binding domain, a small GTPase involved in vesicle transport and membrane trafficking. Fam71f1 is essential for male fertility. In Fam71f1-mutant mice, the acrosome was abnormally expanded at the round spermatid stage, likely because of enhanced vesicle trafficking. Mass spectrometry analysis after immunoprecipitation indicated that, in testes, FAM71F1 binds not only RAB2B, but also RAB2A. Further study suggested that FAM71F1 binds to the GTP-bound active form of RAB2A/B, but not the inactive form. These results indicate that a complex of FAM71F1 and active RAB2A/B suppresses excessive vesicle trafficking during acrosome formation.
Publications
Morohoshi A, Miyata H, Oyama Y, Oura S, Noda T, Ikawa M. FAM71F1 binds to RAB2A and RAB2B and is essential for acrosome formation and male fertility in mice. Development. 2021 Nov 1;148(21):dev199644. doi: 10.1242/dev.199644. Epub 2021 Oct 29. PMID: 34714330; PMCID: PMC8602946.
Malcher A, Rozwadowska N, Stokowy T, Kolanowski T, Jedrzejczak P, Zietkowiak W, Kurpisz M. Potential biomarkers of nonobstructive azoospermia identified in microarray gene expression analysis. Fertil Steril. 2013 Dec;100(6):1686-94.e1-7. doi: 10.1016/j.fertnstert.2013.07.1999. Epub 2013 Sep 4. PMID: 24012201.
Kolmykov S, Vasiliev G, Osadchuk L, Kleschev M, Osadchuk A. Whole-Exome Sequencing Analysis of Human Semen Quality in Russian Multiethnic Population. Front Genet. 2021 Jun 11;12:662846. doi: 10.3389/fgene.2021.662846. PMID: 34178030; PMCID: PMC8232892.
H2 Gamendazole
Summary
This acid has been shown to prevent the development of new sperm cells.
The Science
H2-Gamendazole is “an orally active anti-spermatogenic indazole caboxylic acid with potent antifertility effects, and no adverse side effects at doses needed to induce reversible infertility.” H2- Gamendazole has been shown to bind and inhibit the activities of HSP90AB1 and EEF1A1. This agent also elicited degradation of the HSP90-dpendent client proteins AKT1 and ERBB2. Gamendazole requires further investigation to maximize the window between effective doses and those that induce side effects, as well as solve issues related to recovery of fertility. Sertoli cells too have been shown to be a target of Gamendazole. (Learn more here.)
Publications
Gupta, V. G., Zelinski, M., Steinmetz, K., Miklos, A., Broward, M., Jakkaraj, S., . . . Tash, J. S. (2011). Anti-Spermatogenic Efficacy of Nonhormonal Male Contraceptive Agent, H2-Gamendazole, in Mice, Rats, Rabbits, and Nonhuman Primates (Rhesus), and a Multiple Low Dose Oral Regimen That Gives 100% Infertility with Complete Reversibility. (Suppl_1), 5-5. Biology of Reproduction,85
Tash, J. S., Attardi, B., Hild, S. A., Chakrasali, R., Jakkaraj, S. R., & Georg, G. I. (2008). A Novel Potent Indazole Carboxylic Acid Derivative Blocks Spermatogenesis and Is Contraceptive in Rats after a Single Oral Dose1.
Tash, J. S., Chakrasali, R., Jakkaraj, S. R., Hughes, J., Smith, S. K., Hornbaker, K., . . . Georg, G. I. (2008). Gamendazole, an Orally Active Indazole Carboxylic Acid Male Contraceptive Agent, Targets HSP90AB1 (HSP90BETA) and EEF1A1 (eEF1A), and Stimulates Il1a Transcription in Rat Sertoli Cells1. Biology of Reproduction,78 (6), 1139-1152
This acid has been shown to prevent the development of new sperm cells.
The Science
H2-Gamendazole is “an orally active anti-spermatogenic indazole caboxylic acid with potent antifertility effects, and no adverse side effects at doses needed to induce reversible infertility.” H2- Gamendazole has been shown to bind and inhibit the activities of HSP90AB1 and EEF1A1. This agent also elicited degradation of the HSP90-dpendent client proteins AKT1 and ERBB2. Gamendazole requires further investigation to maximize the window between effective doses and those that induce side effects, as well as solve issues related to recovery of fertility. Sertoli cells too have been shown to be a target of Gamendazole. (Learn more here.)
Publications
Gupta, V. G., Zelinski, M., Steinmetz, K., Miklos, A., Broward, M., Jakkaraj, S., . . . Tash, J. S. (2011). Anti-Spermatogenic Efficacy of Nonhormonal Male Contraceptive Agent, H2-Gamendazole, in Mice, Rats, Rabbits, and Nonhuman Primates (Rhesus), and a Multiple Low Dose Oral Regimen That Gives 100% Infertility with Complete Reversibility. (Suppl_1), 5-5. Biology of Reproduction,85
Tash, J. S., Attardi, B., Hild, S. A., Chakrasali, R., Jakkaraj, S. R., & Georg, G. I. (2008). A Novel Potent Indazole Carboxylic Acid Derivative Blocks Spermatogenesis and Is Contraceptive in Rats after a Single Oral Dose1.
Tash, J. S., Chakrasali, R., Jakkaraj, S. R., Hughes, J., Smith, S. K., Hornbaker, K., . . . Georg, G. I. (2008). Gamendazole, an Orally Active Indazole Carboxylic Acid Male Contraceptive Agent, Targets HSP90AB1 (HSP90BETA) and EEF1A1 (eEF1A), and Stimulates Il1a Transcription in Rat Sertoli Cells1. Biology of Reproduction,78 (6), 1139-1152
MEIOB / SPATA22 (Meiosis Specific With OB-Fold / Spermatogenesis Associated 22)
Summary
This complex of two proteins binds single-stranded DNA to regulate meiotic recombination.
The Science
MEIOB is a meiosis-specific paralog of RPA1, which is ubiquitously expressed. MEIOB binds specifically to ssDNA in vitro, associates with SPATA22, and localizes as foci on meiotic chromosomes. Inactivation of Meiob or Spata22 leads to infertility in both sexes.
Publications
Xu Y, Greenberg RA, Schonbrunn E, Wang PJ. Meiosis-specific proteins MEIOB and SPATA22 cooperatively associate with the single-stranded DNA-binding replication protein A complex and DNA double-strand breaks. Biol Reprod. 2017;96(5):1096-1104. doi:10.1093/biolre/iox040
Luo, M., Yang, F., Leu, N. et al. MEIOB exhibits single-stranded DNA-binding and exonuclease activities and is essential for meiotic recombination. Nat Commun 4, 2788 (2013). https://doi.org/10.1038/ncomms3788
This complex of two proteins binds single-stranded DNA to regulate meiotic recombination.
The Science
MEIOB is a meiosis-specific paralog of RPA1, which is ubiquitously expressed. MEIOB binds specifically to ssDNA in vitro, associates with SPATA22, and localizes as foci on meiotic chromosomes. Inactivation of Meiob or Spata22 leads to infertility in both sexes.
Publications
Xu Y, Greenberg RA, Schonbrunn E, Wang PJ. Meiosis-specific proteins MEIOB and SPATA22 cooperatively associate with the single-stranded DNA-binding replication protein A complex and DNA double-strand breaks. Biol Reprod. 2017;96(5):1096-1104. doi:10.1093/biolre/iox040
Luo, M., Yang, F., Leu, N. et al. MEIOB exhibits single-stranded DNA-binding and exonuclease activities and is essential for meiotic recombination. Nat Commun 4, 2788 (2013). https://doi.org/10.1038/ncomms3788
MNS1
Summary
MNS1 is expressed in the germ cells of the testes, is essential for assembly of sperm flagella in spermiogenesis, and has been associated with infertility in humans.
The Science
Meiosis-specific nuclear structural protein 1 (MNS1) ia a coiled-coil protein of unknown function, and is essential for spermiogenesis. MNS1 is expressed in the germ cells in the testes and localizes to sperm flagella. MNS1-deficient males are sterile, as they exhibit a sharp reduction in sperm production and the remnant sperm are immotile with abnormal short tails. In MNS1-deficient sperm flagella, the characteristic arrangement of "9+2" microtubules and outer dense fibers are completely disrupted. In addition, MNS1-deficient mice display situs inversus and hydrocephalus. MNS1-deficient tracheal motile cilia lack some outer dynein arms in the axoneme.
Publications
Ta-Shma A, Hjeij R, Perles Z, Dougherty GW, Abu Zahira I, Letteboer SJF, Antony D, Darwish A, Mans DA, Spittler S, Edelbusch C, Cindrić S, Nöthe-Menchen T, Olbrich H, Stuhlmann F, Aprea I, Pennekamp P, Loges NT, Breuer O, Shaag A, Rein AJJT, Gulec EY, Gezdirici A, Abitbul R, Elias N, Amirav I, Schmidts M, Roepman R, Elpeleg O, Omran H. Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility. PLoS Genet. 2018 Aug 27;14(8):e1007602. doi: 10.1371/journal.pgen.1007602. PMID: 30148830; PMCID: PMC6128653.
Zhou J, Yang F, Leu NA, Wang PJ. MNS1 is essential for spermiogenesis and motile ciliary functions in mice. PLoS Genet. 2012;8(3):e1002516. doi: 10.1371/journal.pgen.1002516. Epub 2012 Mar 1. PMID: 22396656; PMCID: PMC3291534.
MNS1 is expressed in the germ cells of the testes, is essential for assembly of sperm flagella in spermiogenesis, and has been associated with infertility in humans.
The Science
Meiosis-specific nuclear structural protein 1 (MNS1) ia a coiled-coil protein of unknown function, and is essential for spermiogenesis. MNS1 is expressed in the germ cells in the testes and localizes to sperm flagella. MNS1-deficient males are sterile, as they exhibit a sharp reduction in sperm production and the remnant sperm are immotile with abnormal short tails. In MNS1-deficient sperm flagella, the characteristic arrangement of "9+2" microtubules and outer dense fibers are completely disrupted. In addition, MNS1-deficient mice display situs inversus and hydrocephalus. MNS1-deficient tracheal motile cilia lack some outer dynein arms in the axoneme.
Publications
Ta-Shma A, Hjeij R, Perles Z, Dougherty GW, Abu Zahira I, Letteboer SJF, Antony D, Darwish A, Mans DA, Spittler S, Edelbusch C, Cindrić S, Nöthe-Menchen T, Olbrich H, Stuhlmann F, Aprea I, Pennekamp P, Loges NT, Breuer O, Shaag A, Rein AJJT, Gulec EY, Gezdirici A, Abitbul R, Elias N, Amirav I, Schmidts M, Roepman R, Elpeleg O, Omran H. Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility. PLoS Genet. 2018 Aug 27;14(8):e1007602. doi: 10.1371/journal.pgen.1007602. PMID: 30148830; PMCID: PMC6128653.
Zhou J, Yang F, Leu NA, Wang PJ. MNS1 is essential for spermiogenesis and motile ciliary functions in mice. PLoS Genet. 2012;8(3):e1002516. doi: 10.1371/journal.pgen.1002516. Epub 2012 Mar 1. PMID: 22396656; PMCID: PMC3291534.
ODF1 (Outer Dense Fiber Of Sperm Tails 1)
Summary
This is the main protein found in the dense fibers of the sperm tail and may be involved in the coupling of the sperm head and tail.
The Science
The main protein of sperm tail outer dense fibers, ODF1/HSPB10, belongs to the family of small heat shock proteins that function as molecular chaperones. Heterozygous mutant male mice are fertile while sperm motility is reduced, but Odf1-deficient male mice are infertile due to the detachment of the sperm head. Although headless tails are somehow motile, transmission electron microscopy reveals a disorganization of the mitochondrial sheath, as well as of the outer dense fibers.
Publications
Yang K, Grzmil P, Meinhardt A, Hoyer-Fender S. Haplo-deficiency of ODF1/HSPB10 in mouse sperm causes relaxation of head-to-tail linkage. Reproduction. 2014 Nov;148(5):499-506. doi: 10.1530/REP-14-0370. Epub 2014 Aug 12. PMID: 25118300.
Yang K, Meinhardt A, Zhang B, Grzmil P, Adham IM, Hoyer-Fender S. The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol Cell Biol. 2012 Jan;32(1):216-25. doi: 10.1128/MCB.06158-11. Epub 2011 Oct 28. PMID: 22037768; PMCID: PMC3255718.
This is the main protein found in the dense fibers of the sperm tail and may be involved in the coupling of the sperm head and tail.
The Science
The main protein of sperm tail outer dense fibers, ODF1/HSPB10, belongs to the family of small heat shock proteins that function as molecular chaperones. Heterozygous mutant male mice are fertile while sperm motility is reduced, but Odf1-deficient male mice are infertile due to the detachment of the sperm head. Although headless tails are somehow motile, transmission electron microscopy reveals a disorganization of the mitochondrial sheath, as well as of the outer dense fibers.
Publications
Yang K, Grzmil P, Meinhardt A, Hoyer-Fender S. Haplo-deficiency of ODF1/HSPB10 in mouse sperm causes relaxation of head-to-tail linkage. Reproduction. 2014 Nov;148(5):499-506. doi: 10.1530/REP-14-0370. Epub 2014 Aug 12. PMID: 25118300.
Yang K, Meinhardt A, Zhang B, Grzmil P, Adham IM, Hoyer-Fender S. The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol Cell Biol. 2012 Jan;32(1):216-25. doi: 10.1128/MCB.06158-11. Epub 2011 Oct 28. PMID: 22037768; PMCID: PMC3255718.
RTI 4587
Summary
This molecule has been shown to reversibly prevent sperm from fully maturing, leading to infertility, without impacting libido or sexual performance.
The Science
Indenopyridines are classified as a group of compounds that selectively perturb Sertoli-germ cell adhesion, causing massive exfoliation of germ cells from the seminiferous epithelium, without harming spermatogonia. They were initially developed as antihistamines. However, the early derivative of hexahydroindenopyridined(4aRS,5SR,9bRS)- 2-ethyl-1,3,4,4a,5,9b-hexahydro-7-methyl-5-p-tolyl-2Hindeno( 1,2-c) pyridine hydrochloride (Sandoz 20-438)d had strong anti- spermatogenic effects in rats, dogs, and mice. There were no detrimental effects on an ability to ejaculate, and the profound anti- spermatogenic effects of the indenopyridines were reversible in most species. Issues surrounding irreversibility establishing efficacy in an animal model have prevented further development. (Learn more here.)
Publications
Pozor, M. A., Macpherson, M. L., Mcdonnell, S. M., Nollin, M., Roser, J. F., Love, C., . . . Troedsson, M. H. (2013). Indenopyride derivative RTI- 4587-073(l): A candidate for male contraception in stallions. Theriogenology,80 (9), 1006-1016.
Pozor, M., Zambrano, G., Roser, J., Hess, R., Runyon, S., Runcan, E., . . . Kelleman, A. (2014). Acute and Chronic Effects of a Contraceptive Compound RTI-4587-073(l) on Testicular Histology and Endocrine Function in Miniature Horse Stallions. Reproduction in Domestic Animals,49(3), 392-402.
This molecule has been shown to reversibly prevent sperm from fully maturing, leading to infertility, without impacting libido or sexual performance.
The Science
Indenopyridines are classified as a group of compounds that selectively perturb Sertoli-germ cell adhesion, causing massive exfoliation of germ cells from the seminiferous epithelium, without harming spermatogonia. They were initially developed as antihistamines. However, the early derivative of hexahydroindenopyridined(4aRS,5SR,9bRS)- 2-ethyl-1,3,4,4a,5,9b-hexahydro-7-methyl-5-p-tolyl-2Hindeno( 1,2-c) pyridine hydrochloride (Sandoz 20-438)d had strong anti- spermatogenic effects in rats, dogs, and mice. There were no detrimental effects on an ability to ejaculate, and the profound anti- spermatogenic effects of the indenopyridines were reversible in most species. Issues surrounding irreversibility establishing efficacy in an animal model have prevented further development. (Learn more here.)
Publications
Pozor, M. A., Macpherson, M. L., Mcdonnell, S. M., Nollin, M., Roser, J. F., Love, C., . . . Troedsson, M. H. (2013). Indenopyride derivative RTI- 4587-073(l): A candidate for male contraception in stallions. Theriogenology,80 (9), 1006-1016.
Pozor, M., Zambrano, G., Roser, J., Hess, R., Runyon, S., Runcan, E., . . . Kelleman, A. (2014). Acute and Chronic Effects of a Contraceptive Compound RTI-4587-073(l) on Testicular Histology and Endocrine Function in Miniature Horse Stallions. Reproduction in Domestic Animals,49(3), 392-402.
SPATA16 (Spermatogenesis Associated 16)
Summary
This gene is associated with globozoospermia, where sperm have absent or malformed acrosomes.
The Science
SPATA16 has been shown to be associated with infertility in humans, and could be developed as a contraceptive target. Variants in SPATA16 cause the malformation of the acrosome, which can be totally absent in most severe cases. Additional features are an abnormal nuclear shape and abnormal arrangement of the mitochondria of the spermatozoon.
Publications
Faja F, Pallotti F, Cargnelutti F, Senofonte G, Carlini T, Lenzi A, Lombardo F, Paoli D. Molecular Analysis of DPY19L2, PICK1 and SPATA16 in Italian Unrelated Globozoospermic Men. Life (Basel). 2021 Jun 30;11(7):641. doi: 10.3390/life11070641. PMID: 34209343; PMCID: PMC8307282.
Behvarz M, Rahmani SA, Siasi Torbati E, Danaei Mehrabad S, Bikhof Torbati M. Association of CATSPER1, SPATA16 and TEX11 genes polymorphism with idiopathic azoospermia and oligospermia risk in Iranian population. BMC Med Genomics. 2022 Mar 5;15(1):47. doi: 10.1186/s12920-022-01197-w. PMID: 35248021; PMCID: PMC8897944.
This gene is associated with globozoospermia, where sperm have absent or malformed acrosomes.
The Science
SPATA16 has been shown to be associated with infertility in humans, and could be developed as a contraceptive target. Variants in SPATA16 cause the malformation of the acrosome, which can be totally absent in most severe cases. Additional features are an abnormal nuclear shape and abnormal arrangement of the mitochondria of the spermatozoon.
Publications
Faja F, Pallotti F, Cargnelutti F, Senofonte G, Carlini T, Lenzi A, Lombardo F, Paoli D. Molecular Analysis of DPY19L2, PICK1 and SPATA16 in Italian Unrelated Globozoospermic Men. Life (Basel). 2021 Jun 30;11(7):641. doi: 10.3390/life11070641. PMID: 34209343; PMCID: PMC8307282.
Behvarz M, Rahmani SA, Siasi Torbati E, Danaei Mehrabad S, Bikhof Torbati M. Association of CATSPER1, SPATA16 and TEX11 genes polymorphism with idiopathic azoospermia and oligospermia risk in Iranian population. BMC Med Genomics. 2022 Mar 5;15(1):47. doi: 10.1186/s12920-022-01197-w. PMID: 35248021; PMCID: PMC8897944.
SPINK2 (Serine Peptidase Inhibitor Kazal Type 2)
Summary
This gene is required to neutralize proteases and protect maturing sperm.
The Science
Recombinant mouse SPINK2 has trypsin-inhibitory activity. Distribution analyses revealed that Spink2 is transcribed strongly in the testis and weakly in the epididymis, but is not detected in other mouse tissues. Expression of Spink2 is specific to germ cells in the testis and is first evident at the pachytene spermatocyte stage. Immunoblot analyses demonstrated that SPINK2 protein is present in male germ cells at all developmental stages, including in testicular spermatogenic cells, testicular sperm, and mature sperm. Mutant male mice exhibit significantly impaired fertility; further phenotypic analyses revealed that testicular integrity is disrupted, resulting in a reduction in sperm number. Testes from mutant mice exhibit abnormal spermatogenesis and germ cell apoptosis accompanied by elevated serine protease activity.
Publications
Lee B, Park I, Jin S, Choi H, Kwon JT, Kim J, Jeong J, Cho BN, Eddy EM, Cho C. Impaired spermatogenesis and fertility in mice carrying a mutation in the Spink2 gene expressed predominantly in testes. J Biol Chem. 2011 Aug 19;286(33):29108-29117. doi: 10.1074/jbc.M111.244905. Epub 2011 Jun 24. PMID: 21705336; PMCID: PMC3190718.
Kherraf ZE, Christou-Kent M, Karaouzene T, Amiri-Yekta A, Martinez G, Vargas AS, Lambert E, Borel C, Dorphin B, Aknin-Seifer I, Mitchell MJ, Metzler-Guillemain C, Escoffier J, Nef S, Grepillat M, Thierry-Mieg N, Satre V, Bailly M, Boitrelle F, Pernet-Gallay K, Hennebicq S, Fauré J, Bottari SP, Coutton C, Ray PF, Arnoult C. SPINK2 deficiency causes infertility by inducing sperm defects in heterozygotes and azoospermia in homozygotes. EMBO Mol Med. 2017 Aug;9(8):1132-1149. doi: 10.15252/emmm.201607461. PMID: 28554943; PMCID: PMC5538632.
This gene is required to neutralize proteases and protect maturing sperm.
The Science
Recombinant mouse SPINK2 has trypsin-inhibitory activity. Distribution analyses revealed that Spink2 is transcribed strongly in the testis and weakly in the epididymis, but is not detected in other mouse tissues. Expression of Spink2 is specific to germ cells in the testis and is first evident at the pachytene spermatocyte stage. Immunoblot analyses demonstrated that SPINK2 protein is present in male germ cells at all developmental stages, including in testicular spermatogenic cells, testicular sperm, and mature sperm. Mutant male mice exhibit significantly impaired fertility; further phenotypic analyses revealed that testicular integrity is disrupted, resulting in a reduction in sperm number. Testes from mutant mice exhibit abnormal spermatogenesis and germ cell apoptosis accompanied by elevated serine protease activity.
Publications
Lee B, Park I, Jin S, Choi H, Kwon JT, Kim J, Jeong J, Cho BN, Eddy EM, Cho C. Impaired spermatogenesis and fertility in mice carrying a mutation in the Spink2 gene expressed predominantly in testes. J Biol Chem. 2011 Aug 19;286(33):29108-29117. doi: 10.1074/jbc.M111.244905. Epub 2011 Jun 24. PMID: 21705336; PMCID: PMC3190718.
Kherraf ZE, Christou-Kent M, Karaouzene T, Amiri-Yekta A, Martinez G, Vargas AS, Lambert E, Borel C, Dorphin B, Aknin-Seifer I, Mitchell MJ, Metzler-Guillemain C, Escoffier J, Nef S, Grepillat M, Thierry-Mieg N, Satre V, Bailly M, Boitrelle F, Pernet-Gallay K, Hennebicq S, Fauré J, Bottari SP, Coutton C, Ray PF, Arnoult C. SPINK2 deficiency causes infertility by inducing sperm defects in heterozygotes and azoospermia in homozygotes. EMBO Mol Med. 2017 Aug;9(8):1132-1149. doi: 10.15252/emmm.201607461. PMID: 28554943; PMCID: PMC5538632.
TBC1D21 (TBC1 Domain Family Member 21)
Summary
This gene is involved in the generation of new sperm and variants of the gene are associated with multiple morphological abnormalities in sperm.
The Science
TBC1D21 is a protein specifically expressed in the testes that exhibits specific localizations of elongating and elongated spermatids during mammalian spermiogenesis. Loss of Tbc1d21 in mice results in male infertility, characterized by defects in sperm tail structure and diminished sperm motility. The mitochondria of the sperm-tail have an abnormal irregular arrangement, abnormal diameter, and structural defects. Additionally, the axoneme structure of sperm tails is disturbed. TBC1D21 interacts with ACTB, TPM3, SPATA19, and VDAC3 to regulate the architecture of the sperm midpiece. Overall, TBC1D21 is a scaffold protein required for organization and stabilization of the mitochondrial sheath morphology.
Publications
Chen Y, Chen X, Zhang H, Sha Y, Meng R, Shao T, Yang X, Jin P, Zhuang Y, Min W, Xu D, Jiang Z, Li Y, Li L, Yue W, Yin C. TBC1D21 is an essential factor for sperm mitochondrial sheath assembly and male fertility. Biol Reprod. 2022 Apr 9:ioac069. doi: 10.1093/biolre/ioac069. Epub ahead of print. PMID: 35403672.
Wang YY, Ke CC, Chen YL, Lin YH, Yu IS, Ku WC, O'Bryan MK, Lin YH. Deficiency of the Tbc1d21 gene causes male infertility with morphological abnormalities of the sperm mitochondria and flagellum in mice. PLoS Genet. 2020 Sep 25;16(9):e1009020. doi: 10.1371/journal.pgen.1009020. PMID: 32976492; PMCID: PMC7549768.
Ke CC, Lin YH, Wang YY, Wu YY, Chen MF, Ku WC, Chiang HS, Lai TH. TBC1D21 Potentially Interacts with and Regulates Rap1 during Murine Spermatogenesis. Int J Mol Sci. 2018 Oct 23;19(11):3292. doi: 10.3390/ijms19113292. PMID: 30360518; PMCID: PMC6274753.
This gene is involved in the generation of new sperm and variants of the gene are associated with multiple morphological abnormalities in sperm.
The Science
TBC1D21 is a protein specifically expressed in the testes that exhibits specific localizations of elongating and elongated spermatids during mammalian spermiogenesis. Loss of Tbc1d21 in mice results in male infertility, characterized by defects in sperm tail structure and diminished sperm motility. The mitochondria of the sperm-tail have an abnormal irregular arrangement, abnormal diameter, and structural defects. Additionally, the axoneme structure of sperm tails is disturbed. TBC1D21 interacts with ACTB, TPM3, SPATA19, and VDAC3 to regulate the architecture of the sperm midpiece. Overall, TBC1D21 is a scaffold protein required for organization and stabilization of the mitochondrial sheath morphology.
Publications
Chen Y, Chen X, Zhang H, Sha Y, Meng R, Shao T, Yang X, Jin P, Zhuang Y, Min W, Xu D, Jiang Z, Li Y, Li L, Yue W, Yin C. TBC1D21 is an essential factor for sperm mitochondrial sheath assembly and male fertility. Biol Reprod. 2022 Apr 9:ioac069. doi: 10.1093/biolre/ioac069. Epub ahead of print. PMID: 35403672.
Wang YY, Ke CC, Chen YL, Lin YH, Yu IS, Ku WC, O'Bryan MK, Lin YH. Deficiency of the Tbc1d21 gene causes male infertility with morphological abnormalities of the sperm mitochondria and flagellum in mice. PLoS Genet. 2020 Sep 25;16(9):e1009020. doi: 10.1371/journal.pgen.1009020. PMID: 32976492; PMCID: PMC7549768.
Ke CC, Lin YH, Wang YY, Wu YY, Chen MF, Ku WC, Chiang HS, Lai TH. TBC1D21 Potentially Interacts with and Regulates Rap1 during Murine Spermatogenesis. Int J Mol Sci. 2018 Oct 23;19(11):3292. doi: 10.3390/ijms19113292. PMID: 30360518; PMCID: PMC6274753.
Sperm Transport
RISUG (Reversible Inhibition of Sperm Under Guidance)
Summary
Reversible inhibition of sperm under guidance (RISUG) is the development name of a male contraceptive injection that blocks sperm from passing through the vas deferens.
The Science
RISUG is a hydrogel, composed of alternating, styrene and maleic anhydride monomers. The mechanism of action of RISUG is not fully understood, however, a some reports point to modes of action that include partial occlusion, complete occlusion, pH lowering, charge effect, sulphur moiety and protein-SMA agglomerate. The anhydrous copolymer, when injected into the vas deferens, hydrolyzes in the presence of water molecules in the spermatic fluid. The formed hydride generates positive change which attracts negatively charged sperms and thus results in membrane charge imbalance. RISUG is in clinical development in India, and Vasalgel, a licensure of RISUG is in preclinical development in the United States. (Learn more here.)
Publications
Lohiya NK, Alam I, Hussain M, Khan SR, Ansari AS. RISUG: an intravasal injectable male contraceptive. Indian J Med Res. 2014 Nov;140 Suppl(Suppl 1):S63-72. PMID: 25673546; PMCID: PMC4345756.
Lohiya NK, Manivannan B, Mishra PK. Ultrastructural changes in the spermatozoa of langur monkeys Presbytis entellus entellus after vas occlusion with styrene maleic anhydride. Contraception. 1998 Feb;57(2):125-32. doi: 10.1016/s0010-7824(98)00011-0. PMID: 9589840.
Reversible inhibition of sperm under guidance (RISUG) is the development name of a male contraceptive injection that blocks sperm from passing through the vas deferens.
The Science
RISUG is a hydrogel, composed of alternating, styrene and maleic anhydride monomers. The mechanism of action of RISUG is not fully understood, however, a some reports point to modes of action that include partial occlusion, complete occlusion, pH lowering, charge effect, sulphur moiety and protein-SMA agglomerate. The anhydrous copolymer, when injected into the vas deferens, hydrolyzes in the presence of water molecules in the spermatic fluid. The formed hydride generates positive change which attracts negatively charged sperms and thus results in membrane charge imbalance. RISUG is in clinical development in India, and Vasalgel, a licensure of RISUG is in preclinical development in the United States. (Learn more here.)
Publications
Lohiya NK, Alam I, Hussain M, Khan SR, Ansari AS. RISUG: an intravasal injectable male contraceptive. Indian J Med Res. 2014 Nov;140 Suppl(Suppl 1):S63-72. PMID: 25673546; PMCID: PMC4345756.
Lohiya NK, Manivannan B, Mishra PK. Ultrastructural changes in the spermatozoa of langur monkeys Presbytis entellus entellus after vas occlusion with styrene maleic anhydride. Contraception. 1998 Feb;57(2):125-32. doi: 10.1016/s0010-7824(98)00011-0. PMID: 9589840.
Sperm Function
ATP2B4 (ATPase Plasma Membrane Calcium Transporting 4)
Summary
Removing or inhibiting this calcium pump leads to immobile sperm and, subsequently, infertile men.
The Science
ATP2B4 (PMCA4) is the predominant isoform expressed in mouse testis and sperm. ATP2B1-4 is a calcium pump that catalyzes the hydrolysis of ATP coupled with the transport of calcium from the cytoplasm to the extracellular space. They play a critical role in intracellular calcium homeostasis by removing calcium ions from eukaryotic cells against very large concentration gradients. There are four isoforms of ATP2B (designated ATP2B1-4) and approximately a dozen splice variants. The expression of different isoforms and splice variants is regulated in a developmental, tissue- and cell type-specific manner, suggesting that these pumps are functionally adapted to the physiological needs of particular cells and tissues. (Learn more here.)
Publications
Calì, T., Brini, M., & Carafoli, E. (2018). The PMCA pumps in genetically determined neuronal pathologies. Neuroscience Letters,663, 2-11.
Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized 'toolkit' of channels, transporters and stores. Hum Reprod Update. 2006 May-Jun;12(3):253-67. doi: 10.1093/humupd/dmi050. Epub 2005 Dec 7. PMID: 16338990.
Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004 Aug 6;279(32):33742-50. doi: 10.1074/jbc.M404628200. Epub 2004 Jun 3. PMID: 15178683.
Removing or inhibiting this calcium pump leads to immobile sperm and, subsequently, infertile men.
The Science
ATP2B4 (PMCA4) is the predominant isoform expressed in mouse testis and sperm. ATP2B1-4 is a calcium pump that catalyzes the hydrolysis of ATP coupled with the transport of calcium from the cytoplasm to the extracellular space. They play a critical role in intracellular calcium homeostasis by removing calcium ions from eukaryotic cells against very large concentration gradients. There are four isoforms of ATP2B (designated ATP2B1-4) and approximately a dozen splice variants. The expression of different isoforms and splice variants is regulated in a developmental, tissue- and cell type-specific manner, suggesting that these pumps are functionally adapted to the physiological needs of particular cells and tissues. (Learn more here.)
Publications
Calì, T., Brini, M., & Carafoli, E. (2018). The PMCA pumps in genetically determined neuronal pathologies. Neuroscience Letters,663, 2-11.
Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized 'toolkit' of channels, transporters and stores. Hum Reprod Update. 2006 May-Jun;12(3):253-67. doi: 10.1093/humupd/dmi050. Epub 2005 Dec 7. PMID: 16338990.
Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004 Aug 6;279(32):33742-50. doi: 10.1074/jbc.M404628200. Epub 2004 Jun 3. PMID: 15178683.
Cinchonine Derivatives
Summary
Cinchona is a genus of flowering plants native to South America, and medicines derived from it have shown to prevent normal sperm function (e.g., impact sperm motility and spermatogenesis).
The Science
Cinchonine is a natural compound present in Cinchona bark. Synthesis of cinchonine derivatives came about as an effort towards identifying non-detergent spermicides acting through interaction with sulfhydryl groups present on sperm cells. Sulfhydryl-disulfide conversion plays an important role in sperm action (mainly in its maturation, motility and viability). (Learn more here.)
Publications
Pandey RR, Srivastava A, Malasoni R, Naqvi A, Jain A, Maikhuri JP, Paliwal S, Gupta G, Dwivedi AK. Synthesis of 3-(1-alkyl/aminoalkyl-3-vinyl-piperidin-4-yl)-1-(quinolin-4-yl)-propan-1-ones and their 2-methylene derivatives as potential spermicidal and microbicidal agents. Bioorg Med Chem Lett. 2012 Sep 1;22(17):5735-8. doi: 10.1016/j.bmcl.2012.06.062. Epub 2012 Jul 3. PMID: 22846917.
Jin ZL, Yan W, Qu M, Ge CZ, Chen X, Zhang SF. Cinchonine activates endoplasmic reticulum stress-induced apoptosis in human liver cancer cells. Exp Ther Med. 2018 Jun;15(6):5046-5050. doi: 10.3892/etm.2018.6005. Epub 2018 Mar 29. PMID: 29805529; PMCID: PMC5952100.
Cinchona is a genus of flowering plants native to South America, and medicines derived from it have shown to prevent normal sperm function (e.g., impact sperm motility and spermatogenesis).
The Science
Cinchonine is a natural compound present in Cinchona bark. Synthesis of cinchonine derivatives came about as an effort towards identifying non-detergent spermicides acting through interaction with sulfhydryl groups present on sperm cells. Sulfhydryl-disulfide conversion plays an important role in sperm action (mainly in its maturation, motility and viability). (Learn more here.)
Publications
Pandey RR, Srivastava A, Malasoni R, Naqvi A, Jain A, Maikhuri JP, Paliwal S, Gupta G, Dwivedi AK. Synthesis of 3-(1-alkyl/aminoalkyl-3-vinyl-piperidin-4-yl)-1-(quinolin-4-yl)-propan-1-ones and their 2-methylene derivatives as potential spermicidal and microbicidal agents. Bioorg Med Chem Lett. 2012 Sep 1;22(17):5735-8. doi: 10.1016/j.bmcl.2012.06.062. Epub 2012 Jul 3. PMID: 22846917.
Jin ZL, Yan W, Qu M, Ge CZ, Chen X, Zhang SF. Cinchonine activates endoplasmic reticulum stress-induced apoptosis in human liver cancer cells. Exp Ther Med. 2018 Jun;15(6):5046-5050. doi: 10.3892/etm.2018.6005. Epub 2018 Mar 29. PMID: 29805529; PMCID: PMC5952100.
CD9 (Cell Differentiation Antigen 9)
Summary
This protein appears to be integral in allowing sperm to fertilize an egg; without it, sperm cannot fuse with an egg.
The Science
CD9 belongs to the tetraspanin family of proteins. A fundamental role of tetraspanins appears to be organizing other proteins into a network of multimolecular membrane microdomains that provides a mechanistic framework for membrane protein signaling: cell activation, proliferation, differentiation, motility, fusion and apoptosis. In Cd9-null mice, sperm-egg binding was normal, but sperm-egg fusion was almost entirely inhibited in mutant eggs; intracellular calcium oscillations, which signals fertilization, is absent in all mutant eggs.
Publications
Kaji K, Oda S, Shikano T et al (2000) The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 24:279–282.
Le Naour F, Rubinstein E, Jasmin C et al (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321.
Yoshida K., Kawano N., Harada Y., Miyado K. (2014) Role of CD9 in Sperm–Egg Fusion and Virus-Induced Cell Fusion in Mammals. In: Sawada H., Inoue N., Iwano M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_31
This protein appears to be integral in allowing sperm to fertilize an egg; without it, sperm cannot fuse with an egg.
The Science
CD9 belongs to the tetraspanin family of proteins. A fundamental role of tetraspanins appears to be organizing other proteins into a network of multimolecular membrane microdomains that provides a mechanistic framework for membrane protein signaling: cell activation, proliferation, differentiation, motility, fusion and apoptosis. In Cd9-null mice, sperm-egg binding was normal, but sperm-egg fusion was almost entirely inhibited in mutant eggs; intracellular calcium oscillations, which signals fertilization, is absent in all mutant eggs.
Publications
Kaji K, Oda S, Shikano T et al (2000) The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 24:279–282.
Le Naour F, Rubinstein E, Jasmin C et al (2000) Severely reduced female fertility in CD9-deficient mice. Science 287:319–321.
Yoshida K., Kawano N., Harada Y., Miyado K. (2014) Role of CD9 in Sperm–Egg Fusion and Virus-Induced Cell Fusion in Mammals. In: Sawada H., Inoue N., Iwano M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_31
CD52g
Summary
CD52g is a carbohydrate epitope on the surface of sperm that is being exploited to develop sperm-agglutinating products.
The Science
CD52g, a glycosylphosphatidylinositol-anchored glycoprotein found in abundance on the surface of human sperm is targeted through monoclonal and polyvalent antibodies. Currently being developed for vaginal delivery, the possibility exists for this unique technology to be exploited for male delivery.
Publications
Baldeon-Vaca G, Marathe JG, Politch JA, Mausser E, Pudney J, Doud J, Nador E, Zeitlin L, Pauly M, Moench TR, Brennan M, Whaley KJ, Anderson DJ. Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine. 2021 Jul;69:103478. doi: 10.1016/j.ebiom.2021.103478. Epub 2021 Jul 10. PMID: 34256345; PMCID: PMC8324805.
Shrestha B, Vincent K, Schaefer A, Zhu Y, Vargas G, Motamedi M, Swope K, Morton J, Simpson C, Pham H, Brennan MB, Pauly MH, Zeitlin L, Bratcher B, Whaley KJ, Moench TR, Lai SK. Hexavalent sperm-binding IgG antibody released from vaginal film for development of potent on-demand nonhormonal female contraception. Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2107832118. doi: 10.1073/pnas.2107832118. PMID: 34815336; PMCID: PMC8640842.
Anderson DJ, Politch JA, Cone RA, Zeitlin L, Lai SK, Santangelo PJ, Moench TR, Whaley KJ. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies†. Biol Reprod. 2020 Aug 4;103(2):275-285. doi: 10.1093/biolre/ioaa096. PMID: 32607584; PMCID: PMC7401387.
CD52g is a carbohydrate epitope on the surface of sperm that is being exploited to develop sperm-agglutinating products.
The Science
CD52g, a glycosylphosphatidylinositol-anchored glycoprotein found in abundance on the surface of human sperm is targeted through monoclonal and polyvalent antibodies. Currently being developed for vaginal delivery, the possibility exists for this unique technology to be exploited for male delivery.
Publications
Baldeon-Vaca G, Marathe JG, Politch JA, Mausser E, Pudney J, Doud J, Nador E, Zeitlin L, Pauly M, Moench TR, Brennan M, Whaley KJ, Anderson DJ. Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine. 2021 Jul;69:103478. doi: 10.1016/j.ebiom.2021.103478. Epub 2021 Jul 10. PMID: 34256345; PMCID: PMC8324805.
Shrestha B, Vincent K, Schaefer A, Zhu Y, Vargas G, Motamedi M, Swope K, Morton J, Simpson C, Pham H, Brennan MB, Pauly MH, Zeitlin L, Bratcher B, Whaley KJ, Moench TR, Lai SK. Hexavalent sperm-binding IgG antibody released from vaginal film for development of potent on-demand nonhormonal female contraception. Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2107832118. doi: 10.1073/pnas.2107832118. PMID: 34815336; PMCID: PMC8640842.
Anderson DJ, Politch JA, Cone RA, Zeitlin L, Lai SK, Santangelo PJ, Moench TR, Whaley KJ. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies†. Biol Reprod. 2020 Aug 4;103(2):275-285. doi: 10.1093/biolre/ioaa096. PMID: 32607584; PMCID: PMC7401387.
CFAP44
Summary
This gene is associated with spermatogenesis as well multiple morphological abnormalities of sperm flagella, and has been shown to be related with infertility in men.
The Science
Cilia and flagella-associated protein 44 (CFAP44) is a flagellar protein involved in sperm flagellum axoneme organization and function. Whole-exome sequencing has revealed clinical evidence of CFAP44-associated infertility and severe flagellar defects. CRISPR/Cas9 created homozygous CFAP43/44 male mice that were infertile and presented severe flagellar defects confirming the human genetic results. Immunoelectron and stimulated-emission-depletion microscopy performed on CFAP43 and CFAP44 orthologs in Trypanosoma brucei evidenced that both proteins are located between the doublet microtubules 5 and 6 and the paraflagellar rod.
Publications
Tang S, Wang X, Li W, Yang X, Li Z, Liu W, Li C, Zhu Z, Wang L, Wang J, Zhang L, Sun X, Zhi E, Wang H, Li H, Jin L, Luo Y, Wang J, Yang S, Zhang F. Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Am J Hum Genet. 2017 Jun 1;100(6):854-864. doi: 10.1016/j.ajhg.2017.04.012. Epub 2017 May 25. PMID: 28552195; PMCID: PMC5473723.
Coutton C, Vargas AS, Amiri-Yekta A, Kherraf ZE, Ben Mustapha SF, Le Tanno P, Wambergue-Legrand C, Karaouzène T, Martinez G, Crouzy S, Daneshipour A, Hosseini SH, Mitchell V, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Deleuze JF, Boland A, Hennebicq S, Satre V, Jouk PS, Thierry-Mieg N, Conne B, Dacheux D, Landrein N, Schmitt A, Stouvenel L, Lorès P, El Khouri E, Bottari SP, Fauré J, Wolf JP, Pernet-Gallay K, Escoffier J, Gourabi H, Robinson DR, Nef S, Dulioust E, Zouari R, Bonhivers M, Touré A, Arnoult C, Ray PF. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun. 2018 Feb 15;9(1):686. doi: 10.1038/s41467-017-02792-7. PMID: 29449551; PMCID: PMC5814398.
This gene is associated with spermatogenesis as well multiple morphological abnormalities of sperm flagella, and has been shown to be related with infertility in men.
The Science
Cilia and flagella-associated protein 44 (CFAP44) is a flagellar protein involved in sperm flagellum axoneme organization and function. Whole-exome sequencing has revealed clinical evidence of CFAP44-associated infertility and severe flagellar defects. CRISPR/Cas9 created homozygous CFAP43/44 male mice that were infertile and presented severe flagellar defects confirming the human genetic results. Immunoelectron and stimulated-emission-depletion microscopy performed on CFAP43 and CFAP44 orthologs in Trypanosoma brucei evidenced that both proteins are located between the doublet microtubules 5 and 6 and the paraflagellar rod.
Publications
Tang S, Wang X, Li W, Yang X, Li Z, Liu W, Li C, Zhu Z, Wang L, Wang J, Zhang L, Sun X, Zhi E, Wang H, Li H, Jin L, Luo Y, Wang J, Yang S, Zhang F. Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Am J Hum Genet. 2017 Jun 1;100(6):854-864. doi: 10.1016/j.ajhg.2017.04.012. Epub 2017 May 25. PMID: 28552195; PMCID: PMC5473723.
Coutton C, Vargas AS, Amiri-Yekta A, Kherraf ZE, Ben Mustapha SF, Le Tanno P, Wambergue-Legrand C, Karaouzène T, Martinez G, Crouzy S, Daneshipour A, Hosseini SH, Mitchell V, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Deleuze JF, Boland A, Hennebicq S, Satre V, Jouk PS, Thierry-Mieg N, Conne B, Dacheux D, Landrein N, Schmitt A, Stouvenel L, Lorès P, El Khouri E, Bottari SP, Fauré J, Wolf JP, Pernet-Gallay K, Escoffier J, Gourabi H, Robinson DR, Nef S, Dulioust E, Zouari R, Bonhivers M, Touré A, Arnoult C, Ray PF. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun. 2018 Feb 15;9(1):686. doi: 10.1038/s41467-017-02792-7. PMID: 29449551; PMCID: PMC5814398.
CXCL12 (C-X-C Motif Chemokine Ligand 12)
Summary
This protein could be a component of sperm cell capacitation, meaning inhibiting it could prevent sperm cells from being able to fertilize an egg.
The Science
CXCL12, also known as SDF1 (Stromal cell-derived factor-1) is a member of the CXC family of chemokines that has been found to be constitutively secreted from the bone marrow stromal cells. To date, CXCL12 is the only natural ligand to be identified for CXCR4 (chemokine CXC motif receptor 4), which is a G-protein coupled receptor. CXCR4 is expressed in the sperm acrosome, though only a percentage of spermatozoa express it. A possible explanation to the small fraction of CXCR4-positive spermatozoa could be the physiological heterogeneity present in this cell population, previously described also for seminal parameters (morphology, motility, etc.) and protamine protein content. SDF1 induces spermatozoa accumulation which could reflect spermatozoa chemotaxis for capacitated human spermatozoa, and that the spermatozoa reaction is substance-specific, since no spermatozoa migration was visible in presence of CXCR4 antagonist. Human spermatozoa respond to SDF1 stimulus without significant increase of acrosome reaction, leading to the hypothesis that SDF1-CXCR4 signaling increases the intracellular calcium, capacitates the cells, but does not induce the acrosome reaction, which if early activated could generate a non-competent fertilizing cell. The SDF1-CXCR4 binding leads to activation of several signaling pathways, inclusive of PLCβ, PYK2, Paxillin, Rho, MLCK and MLC, resulting in diverse biological outcomes like migration. Mobilization of intracellular Ca2+ is also an outcome of this signaling process. Furthermore, CXCL12 is locally produced by luteinizing granulosa cells. It specifically contributes to T lymphocytes recruitment and coordinates with local lymphocytes to increase granu- losa cell survival and embryo quality.
Publications
Ma, W.F., Du, J., Fu, L.P., Fang, R., Chen, H.Y., Cai, S.H., 2009. Phenotypic knockout of CXCR4 by a novel recombinant protein TAT/54R/KDEL inhibits tumors metastasis. Mol Cancer Res 7, 1613-1621.
Wu, B., Chien, E. Y., Mol, C. D., Fenalti, G., Liu, W., Katritch, V., . . . Stevens, R. C. (2010). Structures of the CXCR4 Chemokine GPCR with Small- Molecule and Cyclic Peptide Antagonists. Science, 330(6007), 1066-1071
Veldkamp, C., Ziarek, J., Su, J., Basnet, H., Lennertz, R., Weiner, J., Peterson, F., Baker, J. and Volkman, B. (2009). Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Science, 18(7), pp.1359-1369.
This protein could be a component of sperm cell capacitation, meaning inhibiting it could prevent sperm cells from being able to fertilize an egg.
The Science
CXCL12, also known as SDF1 (Stromal cell-derived factor-1) is a member of the CXC family of chemokines that has been found to be constitutively secreted from the bone marrow stromal cells. To date, CXCL12 is the only natural ligand to be identified for CXCR4 (chemokine CXC motif receptor 4), which is a G-protein coupled receptor. CXCR4 is expressed in the sperm acrosome, though only a percentage of spermatozoa express it. A possible explanation to the small fraction of CXCR4-positive spermatozoa could be the physiological heterogeneity present in this cell population, previously described also for seminal parameters (morphology, motility, etc.) and protamine protein content. SDF1 induces spermatozoa accumulation which could reflect spermatozoa chemotaxis for capacitated human spermatozoa, and that the spermatozoa reaction is substance-specific, since no spermatozoa migration was visible in presence of CXCR4 antagonist. Human spermatozoa respond to SDF1 stimulus without significant increase of acrosome reaction, leading to the hypothesis that SDF1-CXCR4 signaling increases the intracellular calcium, capacitates the cells, but does not induce the acrosome reaction, which if early activated could generate a non-competent fertilizing cell. The SDF1-CXCR4 binding leads to activation of several signaling pathways, inclusive of PLCβ, PYK2, Paxillin, Rho, MLCK and MLC, resulting in diverse biological outcomes like migration. Mobilization of intracellular Ca2+ is also an outcome of this signaling process. Furthermore, CXCL12 is locally produced by luteinizing granulosa cells. It specifically contributes to T lymphocytes recruitment and coordinates with local lymphocytes to increase granu- losa cell survival and embryo quality.
Publications
Ma, W.F., Du, J., Fu, L.P., Fang, R., Chen, H.Y., Cai, S.H., 2009. Phenotypic knockout of CXCR4 by a novel recombinant protein TAT/54R/KDEL inhibits tumors metastasis. Mol Cancer Res 7, 1613-1621.
Wu, B., Chien, E. Y., Mol, C. D., Fenalti, G., Liu, W., Katritch, V., . . . Stevens, R. C. (2010). Structures of the CXCR4 Chemokine GPCR with Small- Molecule and Cyclic Peptide Antagonists. Science, 330(6007), 1066-1071
Veldkamp, C., Ziarek, J., Su, J., Basnet, H., Lennertz, R., Weiner, J., Peterson, F., Baker, J. and Volkman, B. (2009). Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Science, 18(7), pp.1359-1369.
CXCR4 (C-X-C Motif Chemokine Receptor 4)
Summary
This protein could be a component of sperm cell capacitation, meaning inhibiting it could prevent sperm cells from being able to fertilize an egg.
The Science
SDF1 is the natural ligand for CXCR4, and SDF1 is expressed in the endometrium, pre-ovulatory follicles and in the oocyte, and is secreted in the follicular fluid. CXCR4 is expressed in the sperm acrosome, though only a percentage of spermatozoa express it. A possible explanation to the small fraction of CXCR4-positive spermatozoa could be the physiological heterogeneity present in this cell population, previously described also for seminal parameters (morphology, motility, etc.) and protamine protein content. SDF1 induces spermatozoa accumulation which could reflect spermatozoa chemotaxis for capacitated human spermatozoa, and that the spermatozoa reaction is substance-specific, since no spermatozoa migration was visible in presence of CXCR4 antagonist. Studies have shown an intracellular [Ca2+] rise in spermatozoa attracted by SDF1, demonstrating that SDF1 give rise to opening of calcium channels and that one of these is a L-type Voltage-operated calcium channels (VOCC), that is the target of Verapamil. Hyperactivity followed the same trend of spermatozoa accumulation without increase of spermatozoa motility (A + B), supporting the hypothesis that SDF1 could really attract the human spermatozoa. Human spermatozoa respond to SDF1 stimulus without significant increase of acrosome reaction, leading to the hypothesis that SDF1-CXCR4 signaling increases the intracellular calcium, capacitates the cells, but does not induce the acrosome reaction, which if early activated could generate a non-competent fertilizing cell. The SDF1-CXCR4 binding leads to activation of several signaling pathways, inclusive of PLCβ, PYK2, Paxillin, Rho, MLCK and MLC, resulting in diverse biological outcomes like migration. Mobilization of intracellular Ca2+ is also an outcome of this signaling process.
Publications
Pawig, L., Klasen, C., Weber, C., Bernhagen, J., & Noels, H. (2015). Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Frontiers in Immunology,6.
Tachibana, K., Hirota, S., Iizasa, H. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 (1998). https://doi.org/10.1038/31261
Wu, B., Chien, E. Y., Mol, C. D., Fenalti, G., Liu, W., Katritch, V., . . . Stevens, R. C. (2010). Structures of the CXCR4 Chemokine GPCR with Small- Molecule and Cyclic Peptide Antagonists. Science, 330(6007), 1066-1071
This protein could be a component of sperm cell capacitation, meaning inhibiting it could prevent sperm cells from being able to fertilize an egg.
The Science
SDF1 is the natural ligand for CXCR4, and SDF1 is expressed in the endometrium, pre-ovulatory follicles and in the oocyte, and is secreted in the follicular fluid. CXCR4 is expressed in the sperm acrosome, though only a percentage of spermatozoa express it. A possible explanation to the small fraction of CXCR4-positive spermatozoa could be the physiological heterogeneity present in this cell population, previously described also for seminal parameters (morphology, motility, etc.) and protamine protein content. SDF1 induces spermatozoa accumulation which could reflect spermatozoa chemotaxis for capacitated human spermatozoa, and that the spermatozoa reaction is substance-specific, since no spermatozoa migration was visible in presence of CXCR4 antagonist. Studies have shown an intracellular [Ca2+] rise in spermatozoa attracted by SDF1, demonstrating that SDF1 give rise to opening of calcium channels and that one of these is a L-type Voltage-operated calcium channels (VOCC), that is the target of Verapamil. Hyperactivity followed the same trend of spermatozoa accumulation without increase of spermatozoa motility (A + B), supporting the hypothesis that SDF1 could really attract the human spermatozoa. Human spermatozoa respond to SDF1 stimulus without significant increase of acrosome reaction, leading to the hypothesis that SDF1-CXCR4 signaling increases the intracellular calcium, capacitates the cells, but does not induce the acrosome reaction, which if early activated could generate a non-competent fertilizing cell. The SDF1-CXCR4 binding leads to activation of several signaling pathways, inclusive of PLCβ, PYK2, Paxillin, Rho, MLCK and MLC, resulting in diverse biological outcomes like migration. Mobilization of intracellular Ca2+ is also an outcome of this signaling process.
Publications
Pawig, L., Klasen, C., Weber, C., Bernhagen, J., & Noels, H. (2015). Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Frontiers in Immunology,6.
Tachibana, K., Hirota, S., Iizasa, H. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 (1998). https://doi.org/10.1038/31261
Wu, B., Chien, E. Y., Mol, C. D., Fenalti, G., Liu, W., Katritch, V., . . . Stevens, R. C. (2010). Structures of the CXCR4 Chemokine GPCR with Small- Molecule and Cyclic Peptide Antagonists. Science, 330(6007), 1066-1071
DNAH2 (Dynein axonemal heavy chain 2)
Summary
This gene is associated with multiple morphological abnormalities of sperm flagella, and has been shown to be related with infertility in men.
The Science
Dynein axonemal heavy chain 2 (DNAH2) is a microtubule-based molecular motor possessing ATPase activity that can convert the chemical energy of ATP into relative sliding between adjacent microtubule doublets to generate ciliary bending, playing a central role in ciliary beats and sperm motility. Dnah2-mutant mice generated by CRISPR/Cas9 genome editing show Dnah2-null males, but not females, are infertile. Dnah2-null sperm cells display absent, short, bent, coiled, and/or irregular flagella with multiple morphological abnormalities of the flagella, and is essential for multiple steps in sperm flagella formation.
Publications
Hwang JY, Nawaz S, Choi J, et al. Genetic Defects in DNAH2 Underlie Male Infertility With Multiple Morphological Abnormalities of the Sperm Flagella in Humans and Mice. Front Cell Dev Biol. 2021;9:662903. Published 2021 Apr 23. doi:10.3389/fcell.2021.662903
Li Y, Wang Y, Wen Y, Zhang T, Wang X, Jiang C, Zheng R, Zhou F, Chen D, Yang Y, Shen Y. Whole-exome sequencing of a cohort of infertile men reveals novel causative genes in teratozoospermia that are chiefly related to sperm head defects. Hum Reprod. 2021 Dec 27;37(1):152-177. doi: 10.1093/humrep/deab229. PMID: 34791246.
Gao Y, Tian S, Sha Y, Zha X, Cheng H, Wang A, Liu C, Lv M, Ni X, Li Q, Wu H, Tan Q, Tang D, Song B, Ding D, Cong J, Xu Y, Zhou P, Wei Z, Cao Y, Xu Y, Zhang F, He X. Novel bi-allelic variants in DNAH2 cause severe asthenoteratozoospermia with multiple morphological abnormalities of the flagella. Reprod Biomed Online. 2021 May;42(5):963-972. doi: 10.1016/j.rbmo.2021.01.011. Epub 2021 Jan 22. PMID: 33771466.
This gene is associated with multiple morphological abnormalities of sperm flagella, and has been shown to be related with infertility in men.
The Science
Dynein axonemal heavy chain 2 (DNAH2) is a microtubule-based molecular motor possessing ATPase activity that can convert the chemical energy of ATP into relative sliding between adjacent microtubule doublets to generate ciliary bending, playing a central role in ciliary beats and sperm motility. Dnah2-mutant mice generated by CRISPR/Cas9 genome editing show Dnah2-null males, but not females, are infertile. Dnah2-null sperm cells display absent, short, bent, coiled, and/or irregular flagella with multiple morphological abnormalities of the flagella, and is essential for multiple steps in sperm flagella formation.
Publications
Hwang JY, Nawaz S, Choi J, et al. Genetic Defects in DNAH2 Underlie Male Infertility With Multiple Morphological Abnormalities of the Sperm Flagella in Humans and Mice. Front Cell Dev Biol. 2021;9:662903. Published 2021 Apr 23. doi:10.3389/fcell.2021.662903
Li Y, Wang Y, Wen Y, Zhang T, Wang X, Jiang C, Zheng R, Zhou F, Chen D, Yang Y, Shen Y. Whole-exome sequencing of a cohort of infertile men reveals novel causative genes in teratozoospermia that are chiefly related to sperm head defects. Hum Reprod. 2021 Dec 27;37(1):152-177. doi: 10.1093/humrep/deab229. PMID: 34791246.
Gao Y, Tian S, Sha Y, Zha X, Cheng H, Wang A, Liu C, Lv M, Ni X, Li Q, Wu H, Tan Q, Tang D, Song B, Ding D, Cong J, Xu Y, Zhou P, Wei Z, Cao Y, Xu Y, Zhang F, He X. Novel bi-allelic variants in DNAH2 cause severe asthenoteratozoospermia with multiple morphological abnormalities of the flagella. Reprod Biomed Online. 2021 May;42(5):963-972. doi: 10.1016/j.rbmo.2021.01.011. Epub 2021 Jan 22. PMID: 33771466.
MFGE8 (Milk Fat Globule-EGF Factor 8 Protein)
Summary
This secreted protein may be necessary in order for sperm cells to adhere to an egg, which is a necessary step in fertilization. Preventing this protein’s normal functioning could lead to infertility in men.
The Science
Milk fat globule-EGF factor 8 protein (MFGE8), also known as lactadherin, is a protein that in humans is encoded by the MFGE8 gene. MFGE8 may function as a cell adhesion protein to connect smooth muscle to elastic fibers in arteries. An amyloid fragment of MFGE8 known as medin accumulates in the aorta with aging. MFGE8 in the vasculature of adults can induce recovery from ischemia by facilitating angiogenesis. It has been suggested that antagonizing MFGE8-induced angiogenesis could be a way of fighting cancer. MFGE8 contains a phosphatidylserine (PS) binding domain, as well as an Arginine-Glycine-Aspartic acid motif, which enables the binding to integrins. MFGE8 binds PS, which is exposed on the surface of apoptotic cells. Opsonization of the apoptotic cells and binding to integrins on the surface of phagocytic cells, mediates the engulfment of the dead cell.
Publications
Copland, S.D., Murphy, A.A., Shur, B.D., 2009. The mouse gamete adhesin, SED1, is expressed on the surface of acrosome-intact human sperm. Fertil Steril 92, 2014-2019.
Ensslin, M.A., Lyng, R., Raymond, A., Copland, S., Shur, B.D., 2007. Novel gamete receptors that facilitate sperm adhesion to the egg coat. Soc Reprod Fertil Suppl 63, 367-383.
Ensslin, M.A., Shur, B.D., 2003. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 114, 405-417.
This secreted protein may be necessary in order for sperm cells to adhere to an egg, which is a necessary step in fertilization. Preventing this protein’s normal functioning could lead to infertility in men.
The Science
Milk fat globule-EGF factor 8 protein (MFGE8), also known as lactadherin, is a protein that in humans is encoded by the MFGE8 gene. MFGE8 may function as a cell adhesion protein to connect smooth muscle to elastic fibers in arteries. An amyloid fragment of MFGE8 known as medin accumulates in the aorta with aging. MFGE8 in the vasculature of adults can induce recovery from ischemia by facilitating angiogenesis. It has been suggested that antagonizing MFGE8-induced angiogenesis could be a way of fighting cancer. MFGE8 contains a phosphatidylserine (PS) binding domain, as well as an Arginine-Glycine-Aspartic acid motif, which enables the binding to integrins. MFGE8 binds PS, which is exposed on the surface of apoptotic cells. Opsonization of the apoptotic cells and binding to integrins on the surface of phagocytic cells, mediates the engulfment of the dead cell.
Publications
Copland, S.D., Murphy, A.A., Shur, B.D., 2009. The mouse gamete adhesin, SED1, is expressed on the surface of acrosome-intact human sperm. Fertil Steril 92, 2014-2019.
Ensslin, M.A., Lyng, R., Raymond, A., Copland, S., Shur, B.D., 2007. Novel gamete receptors that facilitate sperm adhesion to the egg coat. Soc Reprod Fertil Suppl 63, 367-383.
Ensslin, M.A., Shur, B.D., 2003. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 114, 405-417.
Nutlin 3A
Summary
This small molecule inhibitor could negatively impact sperm motility, Adenosine Triphosphate production, and other sperm functions to effectively make males infertile.
The Science
Decreased expression of ubiquinol-cytochrome-c reductase core protein 2 (UQCRC2) and tyrosine phosphorylation (TYP) proteins was noted in studies following the in vitro treatment of spermatozoa with higher concentrations of Nutlin-3a. Similarly, it has been shown that decreased expression of UQCRC2 is correlated with reduced fertility in bulls and increased production of reactive oxygen species (ROS), which has detrimental effects on overall male fertility. Consequently, TYP is associated with capacitation, changes in sperm motility, zona binding competence, and fertilizing ability, whereas the opposite condition had the reverse effect. In particular, sperm TYP and capacitation are both stimulated by cAMP analogues and phosphodiesterase inhibitors, and are inhibited by protein kinase A (PKA) inhibitors, suggesting that cAMP/PKA signaling pathways are involved in these two processes. Increased concentration of Nutlin-3a was associated with a decrease in [Ca2+]i together with UQCRC2 and tyrosine phosphorylation of sperm proteins. These results offer a realistic support that Nutlin-3a stimulates ion transport through the sperm membrane. Considering such a fact, these observations strongly suggest that spermatozoal ion transport may be partly modulated by Nutlin-3a through the inhibition of UQCRC2 with locally released neurotransmitters, and finally contribute to poor fertility. ATP production was decreased in sperm cells treated with Nutlin- 3a. ATP is essential for the functional and structural integrity of 19S complexes and for the recognition and priming of ubiquitinated proteins destined for proteasomal degradation. Nutiln-3a inhibits the activity of p53 by antagonizing MDM2 and it forms an auto regulatory feedback loop in which p53 activates MDM2 transcription factor and its rapid degradation through the ubiquitin proteolysis pathway.
Publications
Atatreh N, Ghattas MA, Bardaweel SK, Rawashdeh SA, Sorkhy MA. Identification of new inhibitors of Mdm2-p53 interaction via pharmacophore and structure-based virtual screening. Drug Des Devel Ther. 2018;12:3741-3752. Published 2018 Nov 2. doi:10.2147/DDDT.S182444
Shukla, K. K., Kwon, W., Rahman, M. S., Park, Y., You, Y., & Pang, M. (2013). Nutlin-3a Decreases Male Fertility via UQCRC2. PLoS ONE,8(10).
This small molecule inhibitor could negatively impact sperm motility, Adenosine Triphosphate production, and other sperm functions to effectively make males infertile.
The Science
Decreased expression of ubiquinol-cytochrome-c reductase core protein 2 (UQCRC2) and tyrosine phosphorylation (TYP) proteins was noted in studies following the in vitro treatment of spermatozoa with higher concentrations of Nutlin-3a. Similarly, it has been shown that decreased expression of UQCRC2 is correlated with reduced fertility in bulls and increased production of reactive oxygen species (ROS), which has detrimental effects on overall male fertility. Consequently, TYP is associated with capacitation, changes in sperm motility, zona binding competence, and fertilizing ability, whereas the opposite condition had the reverse effect. In particular, sperm TYP and capacitation are both stimulated by cAMP analogues and phosphodiesterase inhibitors, and are inhibited by protein kinase A (PKA) inhibitors, suggesting that cAMP/PKA signaling pathways are involved in these two processes. Increased concentration of Nutlin-3a was associated with a decrease in [Ca2+]i together with UQCRC2 and tyrosine phosphorylation of sperm proteins. These results offer a realistic support that Nutlin-3a stimulates ion transport through the sperm membrane. Considering such a fact, these observations strongly suggest that spermatozoal ion transport may be partly modulated by Nutlin-3a through the inhibition of UQCRC2 with locally released neurotransmitters, and finally contribute to poor fertility. ATP production was decreased in sperm cells treated with Nutlin- 3a. ATP is essential for the functional and structural integrity of 19S complexes and for the recognition and priming of ubiquitinated proteins destined for proteasomal degradation. Nutiln-3a inhibits the activity of p53 by antagonizing MDM2 and it forms an auto regulatory feedback loop in which p53 activates MDM2 transcription factor and its rapid degradation through the ubiquitin proteolysis pathway.
Publications
Atatreh N, Ghattas MA, Bardaweel SK, Rawashdeh SA, Sorkhy MA. Identification of new inhibitors of Mdm2-p53 interaction via pharmacophore and structure-based virtual screening. Drug Des Devel Ther. 2018;12:3741-3752. Published 2018 Nov 2. doi:10.2147/DDDT.S182444
Shukla, K. K., Kwon, W., Rahman, M. S., Park, Y., You, Y., & Pang, M. (2013). Nutlin-3a Decreases Male Fertility via UQCRC2. PLoS ONE,8(10).
OVGP1 (Oviduct-specific Glycoprotein)
Summary
This protein is associated with the areas of sperm related to the acrosome reaction, suggesting its involvement in fertility, as well as the potential for causing infertility by preventing its functioning.
The Science
Oviduct-specific glycoprotein also known as oviductal glycoprotein (OGP) or estrogen-dependent oviduct protein (EGP) or mucin-9 (MUC9) is a protein that in humans is encoded by the OVGP1 gene. Oviduct-specific glycoprotein is a large, carbohydrate-rich, epithelial glycoprotein with numerous O-glycosylation sites located within threonine, serine, and proline-rich tandem repeats. The gene is similar to members of the mucin and the glycosyl hydrolase 18 gene families. Regulation of expression may be estrogen-dependent. Gene expression and protein secretion occur during late follicular development through early cleavage-stage embryonic development. The protein is secreted from non-ciliated oviductal epithelial cells and associates with ovulated oocytes, blastomeres, and spermatozoon acrosomal regions. Beyond the oviduct, OVGP1 is detected in the mouse ovary, testis and epididymis suggesting its roles beyond fertilization. It is not detected in the mouse uterus, cervix, vagina, breast, seminal vesicles and prostate gland OVGP1 is expressed by the surface epithelium of the endometrium at the time of embryo implantation in the mouse. It is required to maintain the receptivity phenotype and trophoblast adhesion, OVGP1 mRNA levels are reduced in the endometrium of women with recurrent implantation failure.
Publications
Lyng, R., Shur, B.D., 2009. Mouse oviduct-specific glycoprotein is an egg-associated ZP3-independent sperm-adhesion ligand. Journal of cell science 122, 3894-3906.
Arias EB, Verhage HG, Jaffe RC (Feb 1995). "Complementary deoxyribonucleic acid cloning and molecular characterization of an estrogen-dependent human oviductal glycoprotein". Biol Reprod. 51 (4): 685–94. doi:10.1095/biolreprod51.4.685. PMID 7819450.
Laheri, Saniya; Modi, Deepak; Bhatt, Purvi (Mar 2017). "Extra-oviductal expression of oviductal glycoprotein 1 in mouse: Detection in testis, epididymis and ovary". Journal of Biosciences. 42 (1): 69–80. doi:10.1007/s12038-016-9657-2. PMID 28229966. S2CID 10274575.
This protein is associated with the areas of sperm related to the acrosome reaction, suggesting its involvement in fertility, as well as the potential for causing infertility by preventing its functioning.
The Science
Oviduct-specific glycoprotein also known as oviductal glycoprotein (OGP) or estrogen-dependent oviduct protein (EGP) or mucin-9 (MUC9) is a protein that in humans is encoded by the OVGP1 gene. Oviduct-specific glycoprotein is a large, carbohydrate-rich, epithelial glycoprotein with numerous O-glycosylation sites located within threonine, serine, and proline-rich tandem repeats. The gene is similar to members of the mucin and the glycosyl hydrolase 18 gene families. Regulation of expression may be estrogen-dependent. Gene expression and protein secretion occur during late follicular development through early cleavage-stage embryonic development. The protein is secreted from non-ciliated oviductal epithelial cells and associates with ovulated oocytes, blastomeres, and spermatozoon acrosomal regions. Beyond the oviduct, OVGP1 is detected in the mouse ovary, testis and epididymis suggesting its roles beyond fertilization. It is not detected in the mouse uterus, cervix, vagina, breast, seminal vesicles and prostate gland OVGP1 is expressed by the surface epithelium of the endometrium at the time of embryo implantation in the mouse. It is required to maintain the receptivity phenotype and trophoblast adhesion, OVGP1 mRNA levels are reduced in the endometrium of women with recurrent implantation failure.
Publications
Lyng, R., Shur, B.D., 2009. Mouse oviduct-specific glycoprotein is an egg-associated ZP3-independent sperm-adhesion ligand. Journal of cell science 122, 3894-3906.
Arias EB, Verhage HG, Jaffe RC (Feb 1995). "Complementary deoxyribonucleic acid cloning and molecular characterization of an estrogen-dependent human oviductal glycoprotein". Biol Reprod. 51 (4): 685–94. doi:10.1095/biolreprod51.4.685. PMID 7819450.
Laheri, Saniya; Modi, Deepak; Bhatt, Purvi (Mar 2017). "Extra-oviductal expression of oviductal glycoprotein 1 in mouse: Detection in testis, epididymis and ovary". Journal of Biosciences. 42 (1): 69–80. doi:10.1007/s12038-016-9657-2. PMID 28229966. S2CID 10274575.
PGK2 (Phosphoglycerate Kinase 2)
Summary
This enzyme is essential for sperm motility, and therefore male fertility; preventing it from its normal functioning can make men infertile.
The Science
PGK2 is an isozyme that catalyzes the first ATP-generating step in the glycolytic pathway. It is encoded by an autosomal retrogene that is expressed only during spermatogenesis. It replaces the ubiquitously expressed phosphoglycerate kinase 1 (PGK1) isozyme following repression of Pgk1 transcription by meiotic sex chromosome inactivation during meiotic prophase and by postmeiotic sex chromatin during spermiogenesis. Based on mouse phenotypes, alternative pathways that bypass the PGK step of glycolysis exist. One of these bypass enzymes, acylphosphatase, is active in mouse sperm, perhaps contributing to phenotypic differences between mice lacking GAPDHS or PGK2. Studies determined that PGK2 is not required for the completion of spermatogenesis, but is essential for sperm motility and male fertility. Like GAPDHS, PGK2 is positioned at a key transition point between the ATP-consuming and ATP-generating steps in the sperm glycolytic pathway. Unlike GAPDHS, however, PGK2 and LDHC are not tightly bound to the fibrous sheath, the cytoskeletal structure that defines the limits of the principal piece.
Publications
Danshina, P. V., Geyer, C. B., Dai, Q., Goulding, E. H., Willis, W. D., Kitto, G. B., . . . Obrien, D. A. (2010). Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice1. Biology of Reproduction,82(1), 136-145.
Liu, X., Zhang, H., Shen, X., Liu, F., Liu, J., & Wang, W. (2015). Characteristics of testis-specific phosphoglycerate kinase 2 and its association with human sperm quality. Human Reproduction.
Sawyer, G. M., Monzingo, A. F., Poteet, E. C., Obrien, D. A., & Robertus, J. D. (2007). X-ray analysis of phosphoglycerate kinase 2, a sperm-specific isoform from Mus musculus. Proteins: Structure, Function, and Bioinformatics,71(3), 1134-1144.
This enzyme is essential for sperm motility, and therefore male fertility; preventing it from its normal functioning can make men infertile.
The Science
PGK2 is an isozyme that catalyzes the first ATP-generating step in the glycolytic pathway. It is encoded by an autosomal retrogene that is expressed only during spermatogenesis. It replaces the ubiquitously expressed phosphoglycerate kinase 1 (PGK1) isozyme following repression of Pgk1 transcription by meiotic sex chromosome inactivation during meiotic prophase and by postmeiotic sex chromatin during spermiogenesis. Based on mouse phenotypes, alternative pathways that bypass the PGK step of glycolysis exist. One of these bypass enzymes, acylphosphatase, is active in mouse sperm, perhaps contributing to phenotypic differences between mice lacking GAPDHS or PGK2. Studies determined that PGK2 is not required for the completion of spermatogenesis, but is essential for sperm motility and male fertility. Like GAPDHS, PGK2 is positioned at a key transition point between the ATP-consuming and ATP-generating steps in the sperm glycolytic pathway. Unlike GAPDHS, however, PGK2 and LDHC are not tightly bound to the fibrous sheath, the cytoskeletal structure that defines the limits of the principal piece.
Publications
Danshina, P. V., Geyer, C. B., Dai, Q., Goulding, E. H., Willis, W. D., Kitto, G. B., . . . Obrien, D. A. (2010). Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice1. Biology of Reproduction,82(1), 136-145.
Liu, X., Zhang, H., Shen, X., Liu, F., Liu, J., & Wang, W. (2015). Characteristics of testis-specific phosphoglycerate kinase 2 and its association with human sperm quality. Human Reproduction.
Sawyer, G. M., Monzingo, A. F., Poteet, E. C., Obrien, D. A., & Robertus, J. D. (2007). X-ray analysis of phosphoglycerate kinase 2, a sperm-specific isoform from Mus musculus. Proteins: Structure, Function, and Bioinformatics,71(3), 1134-1144.
QRICH2
Summary
This protein plays a role in sperm flagellar formation and could be targeted by drugs to have a reversible contraceptive effect.
The Science
QRICH2 is a glutamine-rich protein and mutations in QRICH2 have been associated with infertility in men. QRICH has been found to co-localize with sperm flagellar components and regulates a number of proteins involved in sperm flagellar development as well as energy metabolism.
Publications
Zhang G, Li D, Tu C, Meng L, Tan Y, Ji Z, Cheng J, Lu G, Lin G, Zhang H, Sun J, Wang M, Du J, Xu W. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum Mol Genet. 2021 Dec 27;31(2):219-231. doi: 10.1093/hmg/ddab234. PMID: 34415320.
Shen, Y., Zhang, F., Li, F. et al. Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat Commun 10, 433 (2019). https://doi.org/10.1038/s41467-018-08182-x
This protein plays a role in sperm flagellar formation and could be targeted by drugs to have a reversible contraceptive effect.
The Science
QRICH2 is a glutamine-rich protein and mutations in QRICH2 have been associated with infertility in men. QRICH has been found to co-localize with sperm flagellar components and regulates a number of proteins involved in sperm flagellar development as well as energy metabolism.
Publications
Zhang G, Li D, Tu C, Meng L, Tan Y, Ji Z, Cheng J, Lu G, Lin G, Zhang H, Sun J, Wang M, Du J, Xu W. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum Mol Genet. 2021 Dec 27;31(2):219-231. doi: 10.1093/hmg/ddab234. PMID: 34415320.
Shen, Y., Zhang, F., Li, F. et al. Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat Commun 10, 433 (2019). https://doi.org/10.1038/s41467-018-08182-x
SLC9B1 (Solute Carrier Family 9 Member B1)
Summary
This protein plays a role in sperm maturation, motility, and capacitation, so inhibiting it could lead to male infertility.
The Science
SLC9B1 is a sodium/hydrogen exchanger and a transmembrane protein. An increase in bicarbonate (HCO3−) during the transit from the epididymis to the female genital tract activates soluble adenylyl cyclase (sAC). HCO3−-induced cAMP synthesis by sAC stimulates PKA and promotes sperm maturation, motility and capacitation in the female genital tract. Based on knockout evidence, together with previous in vitro studies, it is inferred that NHA1 and NHA2 are functional Na+/H+ exchangers, and thus, Nha1 and Nha2 deficiency causes sperm pHi reduction and immotility via attenuating sAC-mediated cAMP synthesis. However, how NHA1 and NHA2 regulate sAC and the exact correlation between pHi and cAMP level are still unclear. Collectively, Nha1 and Nha2 may be functionally redundant and that they function as key sodium-hydrogen exchangers responsible for sperm motility after leaving the cauda epididymis.
Publications
Anderegg MA, Gyimesi G, Ho TM, Hediger MA, Fuster DG. The Less Well-Known Little Brothers: The SLC9B/NHA Sodium Proton Exchanger Subfamily-Structure, Function, Regulation and Potential Drug-Target Approaches. Front Physiol. 2022 May 25;13:898508. doi: 10.3389/fphys.2022.898508. PMID: 35694410; PMCID: PMC9174904.
Chen, SR., Chen, M., Deng, SL. et al. Sodium–hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis 7, e2152 (2016). https://doi.org/10.1038/cddis.2016.65
Kumar PL, James PF. Identification and characterization of methylation-dependent/independent DNA regulatory elements in the human SLC9B1 gene. Gene. 2015 May 1;561(2):235-48. doi: 10.1016/j.gene.2015.02.050. Epub 2015 Feb 19. PMID: 25701605; PMCID: PMC4361323.
Ye G, Chen C, Han D, Xiong X, Kong Y, Wan B, Yu L. Cloning of a novel human NHEDC1 (Na+/H+ exchanger like domain containing 1) gene expressed specifically in testis. Mol Biol Rep. 2006 Sep;33(3):175-80. doi: 10.1007/s11033-006-0010-y. PMID: 16850186
This protein plays a role in sperm maturation, motility, and capacitation, so inhibiting it could lead to male infertility.
The Science
SLC9B1 is a sodium/hydrogen exchanger and a transmembrane protein. An increase in bicarbonate (HCO3−) during the transit from the epididymis to the female genital tract activates soluble adenylyl cyclase (sAC). HCO3−-induced cAMP synthesis by sAC stimulates PKA and promotes sperm maturation, motility and capacitation in the female genital tract. Based on knockout evidence, together with previous in vitro studies, it is inferred that NHA1 and NHA2 are functional Na+/H+ exchangers, and thus, Nha1 and Nha2 deficiency causes sperm pHi reduction and immotility via attenuating sAC-mediated cAMP synthesis. However, how NHA1 and NHA2 regulate sAC and the exact correlation between pHi and cAMP level are still unclear. Collectively, Nha1 and Nha2 may be functionally redundant and that they function as key sodium-hydrogen exchangers responsible for sperm motility after leaving the cauda epididymis.
Publications
Anderegg MA, Gyimesi G, Ho TM, Hediger MA, Fuster DG. The Less Well-Known Little Brothers: The SLC9B/NHA Sodium Proton Exchanger Subfamily-Structure, Function, Regulation and Potential Drug-Target Approaches. Front Physiol. 2022 May 25;13:898508. doi: 10.3389/fphys.2022.898508. PMID: 35694410; PMCID: PMC9174904.
Chen, SR., Chen, M., Deng, SL. et al. Sodium–hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis 7, e2152 (2016). https://doi.org/10.1038/cddis.2016.65
Kumar PL, James PF. Identification and characterization of methylation-dependent/independent DNA regulatory elements in the human SLC9B1 gene. Gene. 2015 May 1;561(2):235-48. doi: 10.1016/j.gene.2015.02.050. Epub 2015 Feb 19. PMID: 25701605; PMCID: PMC4361323.
Ye G, Chen C, Han D, Xiong X, Kong Y, Wan B, Yu L. Cloning of a novel human NHEDC1 (Na+/H+ exchanger like domain containing 1) gene expressed specifically in testis. Mol Biol Rep. 2006 Sep;33(3):175-80. doi: 10.1007/s11033-006-0010-y. PMID: 16850186
SPATA19 (Spermatogenesis-associated 19)
Summary
It is believed that this is required for the development of sperm, specifically in developing the midpiece of the sperm, necessary for sperm motility.
The Science
SPATA19 is required for mitochondrial architecture and function and for the integrity in the sperm midpiece. Epididymal Spata19-cKOsperm possessed seriously disrupted midpieces, leading to a severe reduction in sperm motility and infertility. One possible reason for this defect is the reduction in mitochondrial COX1 and COX4, two key factors for the biogenesis of the mitochondrial respiratory chain complex IV, which would further reduce activity of the mitochondrial respiratory chain and production of ATP in sperm. It is suggested that SPATA19 plays an important role in the formation of cytochrome c oxidase via regulated expression of COX1 and COX4, either directly or indirectly.
Publications
Mi Y, Shi Z, Li J. Spata19 is critical for sperm mitochondrial function and male fertility. Mol Reprod Dev. 2015 Nov;82(11):907-13. doi: 10.1002/mrd.22536. Epub 2015 Aug 24. PMID: 26265198.
Wang JY, Lan J, Zhao J, Chen L, Liu Y. Molecular characterization, polymorphism and association of porcine SPATA19 gene. Mol Biol Rep. 2012 Oct;39(10):9741-6. doi: 10.1007/s11033-012-1839-x. Epub 2012 Jun 27. PMID: 22736110.
Suzuki-Toyota F, Ito C, Toyama Y, Maekawa M, Yao R, Noda T, Iida H, Toshimori K. Factors maintaining normal sperm tail structure during epididymal maturation studied in Gopc-/- mice. Biol Reprod. 2007 Jul;77(1):71-82. doi: 10.1095/biolreprod.106.058735. Epub 2007 Mar 14. PMID: 17360959.
It is believed that this is required for the development of sperm, specifically in developing the midpiece of the sperm, necessary for sperm motility.
The Science
SPATA19 is required for mitochondrial architecture and function and for the integrity in the sperm midpiece. Epididymal Spata19-cKOsperm possessed seriously disrupted midpieces, leading to a severe reduction in sperm motility and infertility. One possible reason for this defect is the reduction in mitochondrial COX1 and COX4, two key factors for the biogenesis of the mitochondrial respiratory chain complex IV, which would further reduce activity of the mitochondrial respiratory chain and production of ATP in sperm. It is suggested that SPATA19 plays an important role in the formation of cytochrome c oxidase via regulated expression of COX1 and COX4, either directly or indirectly.
Publications
Mi Y, Shi Z, Li J. Spata19 is critical for sperm mitochondrial function and male fertility. Mol Reprod Dev. 2015 Nov;82(11):907-13. doi: 10.1002/mrd.22536. Epub 2015 Aug 24. PMID: 26265198.
Wang JY, Lan J, Zhao J, Chen L, Liu Y. Molecular characterization, polymorphism and association of porcine SPATA19 gene. Mol Biol Rep. 2012 Oct;39(10):9741-6. doi: 10.1007/s11033-012-1839-x. Epub 2012 Jun 27. PMID: 22736110.
Suzuki-Toyota F, Ito C, Toyama Y, Maekawa M, Yao R, Noda T, Iida H, Toshimori K. Factors maintaining normal sperm tail structure during epididymal maturation studied in Gopc-/- mice. Biol Reprod. 2007 Jul;77(1):71-82. doi: 10.1095/biolreprod.106.058735. Epub 2007 Mar 14. PMID: 17360959.
TBC1D21
Summary
This protein is important for formation of the mitochondrial sheath in maturing sperm, and could be targeted to induce reversible contraceptive effects in sperm.
The Science
TBC1D21 is a GTP-ase activating protein and collaborates with numerous proteins to regulate the formation of the sperm mitochondrial sheath. The protein is highly expressed in the testes, and acts as a scaffold protein to regulate the architecture of the sperm midpiece.
Publications
Chen Y, Chen X, Zhang H, Sha Y, Meng R, Shao T, Yang X, Jin P, Zhuang Y, Min W, Xu D, Jiang Z, Li Y, Li L, Yue W, Yin C. TBC1D21 is an essential factor for sperm mitochondrial sheath assembly and male fertility. Biol Reprod. 2022 Apr 9:ioac069. doi: 10.1093/biolre/ioac069. Epub ahead of print. PMID: 35403672.
Wang YY, Ke CC, Chen YL, Lin YH, Yu IS, Ku WC, O'Bryan MK, Lin YH. Deficiency of the Tbc1d21 gene causes male infertility with morphological abnormalities of the sperm mitochondria and flagellum in mice. PLoS Genet. 2020 Sep 25;16(9):e1009020. doi: 10.1371/journal.pgen.1009020. PMID: 32976492; PMCID: PMC7549768.
This protein is important for formation of the mitochondrial sheath in maturing sperm, and could be targeted to induce reversible contraceptive effects in sperm.
The Science
TBC1D21 is a GTP-ase activating protein and collaborates with numerous proteins to regulate the formation of the sperm mitochondrial sheath. The protein is highly expressed in the testes, and acts as a scaffold protein to regulate the architecture of the sperm midpiece.
Publications
Chen Y, Chen X, Zhang H, Sha Y, Meng R, Shao T, Yang X, Jin P, Zhuang Y, Min W, Xu D, Jiang Z, Li Y, Li L, Yue W, Yin C. TBC1D21 is an essential factor for sperm mitochondrial sheath assembly and male fertility. Biol Reprod. 2022 Apr 9:ioac069. doi: 10.1093/biolre/ioac069. Epub ahead of print. PMID: 35403672.
Wang YY, Ke CC, Chen YL, Lin YH, Yu IS, Ku WC, O'Bryan MK, Lin YH. Deficiency of the Tbc1d21 gene causes male infertility with morphological abnormalities of the sperm mitochondria and flagellum in mice. PLoS Genet. 2020 Sep 25;16(9):e1009020. doi: 10.1371/journal.pgen.1009020. PMID: 32976492; PMCID: PMC7549768.
TCTE1 (T-Complex-Associated-Testis-Expressed 1)
Summary
This component of a regulatory complex is a key player of flagellar motility, required for sperm function and humans with defects in this gene have presented with infertility.
The Science
The N-DRC (Nexin-dynein Regulatory Complex) complex is the motor regulating device in the flagellum, which is found in most eukaryotic organisms with flagellum. The deletion of TCTE1 (T-Complex-Associated Testis-Expressed 1), a component of the N-DRC complex also known as DRC5 (Dynein regulatory complex subunit 5), has been shown to cause asthenospermia in mice. TCTE1 is testis-enriched, with its mRNA appearing in early round spermatids and protein localized to the flagellum. TCTE1 is 498 aa in length with a leucine rich repeat domain at the C terminus and is present in eukaryotes containing a flagellum. Knockout of Tcte1 results in male sterility because Tcte1-null spermatozoa show aberrant motility. Although the axoneme is structurally normal in Tcte1 mutant spermatozoa, Tcte1-null sperm demonstrate a significant decrease of ATP, which is used by dynein motors to generate the bending force of the flagellum.
Publications
Zhou, S., Wu, H., Zhang, J. et al. Bi-allelic variants in human TCTE1/DRC5 cause asthenospermia and male infertility. Eur J Hum Genet (2022). https://doi.org/10.1038/s41431-022-01095-w
Castaneda JM, Hua R, Miyata H, Oji A, Guo Y, Cheng Y, Zhou T, Guo X, Cui Y, Shen B, Wang Z, Hu Z, Zhou Z, Sha J, Prunskaite-Hyyrylainen R, Yu Z, Ramirez-Solis R, Ikawa M, Matzuk MM, Liu M. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci USA [Internet]. 2017 Jul 3 [cited 2022 Apr 25];114(27). Available from: https://pnas.org/doi/full/10.1073/pnas.1621279114
This component of a regulatory complex is a key player of flagellar motility, required for sperm function and humans with defects in this gene have presented with infertility.
The Science
The N-DRC (Nexin-dynein Regulatory Complex) complex is the motor regulating device in the flagellum, which is found in most eukaryotic organisms with flagellum. The deletion of TCTE1 (T-Complex-Associated Testis-Expressed 1), a component of the N-DRC complex also known as DRC5 (Dynein regulatory complex subunit 5), has been shown to cause asthenospermia in mice. TCTE1 is testis-enriched, with its mRNA appearing in early round spermatids and protein localized to the flagellum. TCTE1 is 498 aa in length with a leucine rich repeat domain at the C terminus and is present in eukaryotes containing a flagellum. Knockout of Tcte1 results in male sterility because Tcte1-null spermatozoa show aberrant motility. Although the axoneme is structurally normal in Tcte1 mutant spermatozoa, Tcte1-null sperm demonstrate a significant decrease of ATP, which is used by dynein motors to generate the bending force of the flagellum.
Publications
Zhou, S., Wu, H., Zhang, J. et al. Bi-allelic variants in human TCTE1/DRC5 cause asthenospermia and male infertility. Eur J Hum Genet (2022). https://doi.org/10.1038/s41431-022-01095-w
Castaneda JM, Hua R, Miyata H, Oji A, Guo Y, Cheng Y, Zhou T, Guo X, Cui Y, Shen B, Wang Z, Hu Z, Zhou Z, Sha J, Prunskaite-Hyyrylainen R, Yu Z, Ramirez-Solis R, Ikawa M, Matzuk MM, Liu M. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci USA [Internet]. 2017 Jul 3 [cited 2022 Apr 25];114(27). Available from: https://pnas.org/doi/full/10.1073/pnas.1621279114
TRPCs (Transient Receptor Potential Channels)
Summary
These channels could play a role in facilitating the acrosome reaction, a necessary step for sperm to fertilize an egg. Blocking these channels could therefore lead to male infertility.
The Science
The TRP superfamily includes >20 related cation channels that play critical roles in processes ranging from sensory physiology to vasorelaxation and male fertility. How TRP channels are activated, and what their functions might be, are complex and as yet still open questions. In mice, the acrosome reaction is triggered by ZP3, causing a transient Ca2+ influx into sperm through voltage-gated T-type Ca2+ channels. This initial response promotes a sustained increase in Ca2+i that drives the acrosome reaction. TRPC2 has been proposed to participate in the sustained sperm Ca2+ influx triggered by ZP3. Unexpectedly, genetic ablation of mTRPC2 did not compromise the fertility of null mice. These findings suggest that the ion channel involved in the sustained increase in Ca2+i necessary for the AR may be a tetramer composed of different TRPC proteins.
Publications
Montell, C., Birnbaumer, L., & Flockerzi, V. (2002). The TRP Channels, a Remarkably Functional Family. Cell,108(5), 595-598.
Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002 May 28;99(11):7461-6. doi: 10.1073/pnas.102596199. PMID: 12032305; PMCID: PMC124253.
Darszon A, Nishigaki T, Wood C, Treviño CL, Felix R, Beltrán C. Calcium channels and Ca2+ fluctuations in sperm physiology. Int Rev Cytol. 2005;243:79-172. doi: 10.1016/S0074-7696(05)43002-8. PMID: 15797459
These channels could play a role in facilitating the acrosome reaction, a necessary step for sperm to fertilize an egg. Blocking these channels could therefore lead to male infertility.
The Science
The TRP superfamily includes >20 related cation channels that play critical roles in processes ranging from sensory physiology to vasorelaxation and male fertility. How TRP channels are activated, and what their functions might be, are complex and as yet still open questions. In mice, the acrosome reaction is triggered by ZP3, causing a transient Ca2+ influx into sperm through voltage-gated T-type Ca2+ channels. This initial response promotes a sustained increase in Ca2+i that drives the acrosome reaction. TRPC2 has been proposed to participate in the sustained sperm Ca2+ influx triggered by ZP3. Unexpectedly, genetic ablation of mTRPC2 did not compromise the fertility of null mice. These findings suggest that the ion channel involved in the sustained increase in Ca2+i necessary for the AR may be a tetramer composed of different TRPC proteins.
Publications
Montell, C., Birnbaumer, L., & Flockerzi, V. (2002). The TRP Channels, a Remarkably Functional Family. Cell,108(5), 595-598.
Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002 May 28;99(11):7461-6. doi: 10.1073/pnas.102596199. PMID: 12032305; PMCID: PMC124253.
Darszon A, Nishigaki T, Wood C, Treviño CL, Felix R, Beltrán C. Calcium channels and Ca2+ fluctuations in sperm physiology. Int Rev Cytol. 2005;243:79-172. doi: 10.1016/S0074-7696(05)43002-8. PMID: 15797459
Multiple Categories
ACRVI (Acrosomal Vesicle Protein 1)
Summary
Inhibiting this protein has been shown to prevent sperm from attaching to, and penetrating, the protective outer shell of the egg, thereby preventing fertilization.
The Science
ACRV1 is an intra-acrosomal protein known to be conserved across multiple species including humans, mice, and macaques. After the acrosome reaction, ACRV1 remains associated with the inner acrosomal membrane and equatorial segment. The WHO Taskforce on Contraceptive Vaccines designated SP-10 a ‘primary vaccine candidate’ because of its tissue specificity and evidence that the MHS-10 monoclonal antibody inhibits the penetration of zona-free hamster eggs by human spermatozoa. Another monoclonal antibody against ACRV1 inhibited in vitro fertilization by blocking sperm attachment to and penetration of the zona pellucida in mice, pigs and humans. It is speculated that antibodies against ACRV1 prevent dispersal of the acrosomal matrix and therefore the acrosome reaction should remain arrested at some point between fusion pore formation and loss of the acrosomal matrix. (Learn more here.)
Publications
Coonrod, S. A., Herr, J. C., & Westhusin, M. E. (1996). Inhibition of bovine fertilization in vitro by antibodies to SP-10. Reproduction,107(2), 287- 297.
Goyal, S., Manivannan, B., Kumraj, G. R., Ansari, A. S., & Lohiya, N. K. (2013). Evaluation of Efficacy and Safety of Recombinant Sperm-Specific Contraceptive Vaccine in Albino Mice. American Journal of Reproductive Immunology, 69 (5), 495-508
Inhibiting this protein has been shown to prevent sperm from attaching to, and penetrating, the protective outer shell of the egg, thereby preventing fertilization.
The Science
ACRV1 is an intra-acrosomal protein known to be conserved across multiple species including humans, mice, and macaques. After the acrosome reaction, ACRV1 remains associated with the inner acrosomal membrane and equatorial segment. The WHO Taskforce on Contraceptive Vaccines designated SP-10 a ‘primary vaccine candidate’ because of its tissue specificity and evidence that the MHS-10 monoclonal antibody inhibits the penetration of zona-free hamster eggs by human spermatozoa. Another monoclonal antibody against ACRV1 inhibited in vitro fertilization by blocking sperm attachment to and penetration of the zona pellucida in mice, pigs and humans. It is speculated that antibodies against ACRV1 prevent dispersal of the acrosomal matrix and therefore the acrosome reaction should remain arrested at some point between fusion pore formation and loss of the acrosomal matrix. (Learn more here.)
Publications
Coonrod, S. A., Herr, J. C., & Westhusin, M. E. (1996). Inhibition of bovine fertilization in vitro by antibodies to SP-10. Reproduction,107(2), 287- 297.
Goyal, S., Manivannan, B., Kumraj, G. R., Ansari, A. S., & Lohiya, N. K. (2013). Evaluation of Efficacy and Safety of Recombinant Sperm-Specific Contraceptive Vaccine in Albino Mice. American Journal of Reproductive Immunology, 69 (5), 495-508
ADAM2 (ADAM Metallopeptidase Domain 2)
Summary
This protein plays a yet-to-be-determined role in sperm maturation, meaning that inhibiting its function could lead to infertility.
The Science
ADAM2 is a sperm surface membrane protein that may be involved in a variety of biological processes involving cell-cell and cell-matrix interactions. The sperm surface protein fertilin, a member of the ADAM family, is a heterodimer composed of α and β subunits. In mice lacking fertilin α, the level of fertilin α precursor was reduced. Fertilin α may be degraded when unable to form a hetero-dimer with β. ADAM2, which was one of the first identified ADAMs, is the best studied ADAM in reproduction. Analysis of guinea pig ADAM2 revealed that the protein is synthesized in testis and processed during sperm maturation. The proteolytic processing of ADAM2 during epididymal maturation of the sperm removes the pro- and metalloprotease domains, leaving the processed form with an N-terminal disintegrin domain. ADAM2 has been found to form diverse ADAM complexes in spermatogenic cells, including the ADAM1A-ADAM2, ADAM1B- ADAM2, ADAM2-ADAM3 and ADAM2-ADAM3-ADAM6 complexes. In addition, other ADAMs, such as ADAM4 and ADAM5, have been suggested to associate with ADAM2. Although ADAM7 is not believed to associate with ADAM2, these two ADAMs have been found to reciprocally regulate one another’s integrity. The previous findings suggest that ADAM2 plays a central role in maintaining the stability of the proteins involved in the above-listed complexes. In humans, ADAM2 has been identified as a 100-kDa protein in the testis, but was not detected in sperm. This is consistent with the failure of ADAM2 identification in the previous proteomic analyses of human sperm. These findings suggest that the reproductive functions of ADAM2 differ between humans and mice. Protein analyses have shown the presence of potential ADAM2 complexes involving yet-unknown proteins in human testis. (Learn more here.)
Publications
Cho, C. (1998). Fertilization Defects in Sperm from Mice Lacking Fertilin. Science,281(5384), 1857-1859.
Cho, C. (2012). Testicular and epididymal ADAMs: Expression and function during fertilization. Nature Reviews Urology,9(10), 550-560.
Seals, D. F. (2003). The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes & Development,17(1), 7-30.
This protein plays a yet-to-be-determined role in sperm maturation, meaning that inhibiting its function could lead to infertility.
The Science
ADAM2 is a sperm surface membrane protein that may be involved in a variety of biological processes involving cell-cell and cell-matrix interactions. The sperm surface protein fertilin, a member of the ADAM family, is a heterodimer composed of α and β subunits. In mice lacking fertilin α, the level of fertilin α precursor was reduced. Fertilin α may be degraded when unable to form a hetero-dimer with β. ADAM2, which was one of the first identified ADAMs, is the best studied ADAM in reproduction. Analysis of guinea pig ADAM2 revealed that the protein is synthesized in testis and processed during sperm maturation. The proteolytic processing of ADAM2 during epididymal maturation of the sperm removes the pro- and metalloprotease domains, leaving the processed form with an N-terminal disintegrin domain. ADAM2 has been found to form diverse ADAM complexes in spermatogenic cells, including the ADAM1A-ADAM2, ADAM1B- ADAM2, ADAM2-ADAM3 and ADAM2-ADAM3-ADAM6 complexes. In addition, other ADAMs, such as ADAM4 and ADAM5, have been suggested to associate with ADAM2. Although ADAM7 is not believed to associate with ADAM2, these two ADAMs have been found to reciprocally regulate one another’s integrity. The previous findings suggest that ADAM2 plays a central role in maintaining the stability of the proteins involved in the above-listed complexes. In humans, ADAM2 has been identified as a 100-kDa protein in the testis, but was not detected in sperm. This is consistent with the failure of ADAM2 identification in the previous proteomic analyses of human sperm. These findings suggest that the reproductive functions of ADAM2 differ between humans and mice. Protein analyses have shown the presence of potential ADAM2 complexes involving yet-unknown proteins in human testis. (Learn more here.)
Publications
Cho, C. (1998). Fertilization Defects in Sperm from Mice Lacking Fertilin. Science,281(5384), 1857-1859.
Cho, C. (2012). Testicular and epididymal ADAMs: Expression and function during fertilization. Nature Reviews Urology,9(10), 550-560.
Seals, D. F. (2003). The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes & Development,17(1), 7-30.
Adjudin
Summary
Adjudin is a small molecule that has been shown in an animal model to affect spermatogenesis and cause infertility.
The Science
Adjudin has been shown to induce adherens junction disruption between Sertoli cells and round and elongate spermatids in the adult rat (Rattus norvegicus). Adhesion between Sertoli cells and spermatocytes is also affected, but to a lesser extent. Adjudin does not, however, seem to affect blood-testis barrier (BTB) function or perturb adhesion between Sertoli cells and spermatogonia. It also does not perturb cell adhesion in the testes of rats in which a functional apical ectoplasmic specialization (ES) has not yet formed (for example, at 15–25 d of age). Furthermore, histological analyses have shown that cell adhesion in other organs such as kidney, liver, brain, heart, epididymis, prostate and seminal vesicles is not affected. These results seemingly show that Adjudin’s target is the apical ES. Development of adjudin has been stalled due to questions around germline effects and subchronic toxicity. (Learn more here.)
Publications
Cheng, C. Y., Lie, P. P., Wong, E. W., Mruk, D. D., & Silvestrini, B. (2011). Adjudin disrupts spermatogenesis via the action of some unlikely partners. Spermatogenesis,1(4), 291-297.
Li, K., Ni, Y., He, Y., Chen, W., Lu, J., Cheng, C. Y., . . . Shi, Q. (2012). Inhibition of sperm capacitation and fertilizing capacity by adjudin is mediated by chloride and its channels in humans. Human Reproduction,28(1), 47-59.
Mruk, D. D., Wong, C., Silvestrini, B., & Cheng, C. Y. (2006). A male contraceptive targeting germ cell adhesion. Nature Medicine,12(11), 1323-1328.
Adjudin is a small molecule that has been shown in an animal model to affect spermatogenesis and cause infertility.
The Science
Adjudin has been shown to induce adherens junction disruption between Sertoli cells and round and elongate spermatids in the adult rat (Rattus norvegicus). Adhesion between Sertoli cells and spermatocytes is also affected, but to a lesser extent. Adjudin does not, however, seem to affect blood-testis barrier (BTB) function or perturb adhesion between Sertoli cells and spermatogonia. It also does not perturb cell adhesion in the testes of rats in which a functional apical ectoplasmic specialization (ES) has not yet formed (for example, at 15–25 d of age). Furthermore, histological analyses have shown that cell adhesion in other organs such as kidney, liver, brain, heart, epididymis, prostate and seminal vesicles is not affected. These results seemingly show that Adjudin’s target is the apical ES. Development of adjudin has been stalled due to questions around germline effects and subchronic toxicity. (Learn more here.)
Publications
Cheng, C. Y., Lie, P. P., Wong, E. W., Mruk, D. D., & Silvestrini, B. (2011). Adjudin disrupts spermatogenesis via the action of some unlikely partners. Spermatogenesis,1(4), 291-297.
Li, K., Ni, Y., He, Y., Chen, W., Lu, J., Cheng, C. Y., . . . Shi, Q. (2012). Inhibition of sperm capacitation and fertilizing capacity by adjudin is mediated by chloride and its channels in humans. Human Reproduction,28(1), 47-59.
Mruk, D. D., Wong, C., Silvestrini, B., & Cheng, C. Y. (2006). A male contraceptive targeting germ cell adhesion. Nature Medicine,12(11), 1323-1328.
ARMC12 (Armadillo repeat-containing 12)
Summary
This gene regulates mitochondrial dynamics during spermiogenesis and is required for fertility.
The Science
Armadillo repeat-containing 12 (ARMC12) is an essential protein for mitochondrial sheath formation. Absence of ARMC12 causes abnormal mitochondrial coiling along the flagellum, resulting in reduced sperm motility and male sterility. During spermiogenesis, sperm mitochondria in Armc12-null mice cannot elongate properly at the mitochondrial interlocking step which disrupts abnormal mitochondrial coiling. ARMC12 is a mitochondrial peripheral membrane protein and functions as an adherence factor between mitochondria in cultured cells. ARMC12 in testicular germ cells interacts with mitochondrial proteins MIC60, VDAC2, and VDAC3 as well as TBC1D21 and GK2, which are required for mitochondrial sheath formation.
Publications
Liu W, Wei X, Liu X, Chen G, Zhang X, Liang X, Isachenko V, Sha Y, Wang Y. Biallelic mutations in ARMC12 cause asthenozoospermia and multiple midpiece defects in humans and mice. J Med Genet. 2022 May 9:jmedgenet-2021-108137. doi: 10.1136/jmedgenet-2021-108137. Epub ahead of print. PMID: 35534203.
Shimada K, Park S, Miyata H, Yu Z, Morohoshi A, Oura S, Matzuk MM, Ikawa M. ARMC12 regulates spatiotemporal mitochondrial dynamics during spermiogenesis and is required for male fertility. Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2018355118. doi: 10.1073/pnas.2018355118. PMID: 33536340; PMCID: PMC8017931.
Huang Y, Jiang Z, Gao X, Luo P, Jiang X. ARMC Subfamily: Structures, Functions, Evolutions, Interactions, and Diseases. Front Mol Biosci. 2021 Nov 29;8:791597. doi: 10.3389/fmolb.2021.791597. PMID: 34912852; PMCID: PMC8666550.
This gene regulates mitochondrial dynamics during spermiogenesis and is required for fertility.
The Science
Armadillo repeat-containing 12 (ARMC12) is an essential protein for mitochondrial sheath formation. Absence of ARMC12 causes abnormal mitochondrial coiling along the flagellum, resulting in reduced sperm motility and male sterility. During spermiogenesis, sperm mitochondria in Armc12-null mice cannot elongate properly at the mitochondrial interlocking step which disrupts abnormal mitochondrial coiling. ARMC12 is a mitochondrial peripheral membrane protein and functions as an adherence factor between mitochondria in cultured cells. ARMC12 in testicular germ cells interacts with mitochondrial proteins MIC60, VDAC2, and VDAC3 as well as TBC1D21 and GK2, which are required for mitochondrial sheath formation.
Publications
Liu W, Wei X, Liu X, Chen G, Zhang X, Liang X, Isachenko V, Sha Y, Wang Y. Biallelic mutations in ARMC12 cause asthenozoospermia and multiple midpiece defects in humans and mice. J Med Genet. 2022 May 9:jmedgenet-2021-108137. doi: 10.1136/jmedgenet-2021-108137. Epub ahead of print. PMID: 35534203.
Shimada K, Park S, Miyata H, Yu Z, Morohoshi A, Oura S, Matzuk MM, Ikawa M. ARMC12 regulates spatiotemporal mitochondrial dynamics during spermiogenesis and is required for male fertility. Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2018355118. doi: 10.1073/pnas.2018355118. PMID: 33536340; PMCID: PMC8017931.
Huang Y, Jiang Z, Gao X, Luo P, Jiang X. ARMC Subfamily: Structures, Functions, Evolutions, Interactions, and Diseases. Front Mol Biosci. 2021 Nov 29;8:791597. doi: 10.3389/fmolb.2021.791597. PMID: 34912852; PMCID: PMC8666550.
CABYR (Calcium Binding Tyrosine Phosphorylation Regulated Protein)
Summary
This protein is critical in sperm tail development and, therefore, sperm motility; inhibiting its function could prevent sperm from swimming making men infertile.
The Science
CABYR is a protein in the fibrous sheath of the principal piece of the flagellum and is phosphorylated during capacitation. It also appears to be involved in the development of the fibrous sheath during spermiogenesis, and possibly signal transduction and regulation of flagellar energy supply and movements. It also may function as a regulator of both motility- and head- associated functions such as capacitation and the acrosome reaction. Isoform 1 binds calcium in vitro; Isoform 2 and isoform 6 probably bind calcium; Isoform 3 and isoform 5 do not bind calcium in vitro; Isoform 4 probably does not bind calcium. In vitro assays suggest Cabyr-KO spermatozoa cannot penetrate the zona pellucida, but sperm-oolemma interaction isn’t impaired; Cabyr-KO spermatozoa underwent the acrosome reaction, could bind to the zona pellucida (ZP), and could fuse with the oolemma in ZP-free eggs, but they showed a significant loss in the ability to fertilize cumulus-intact and cumulus-free oocytes. Cabyr-KO spermatozoa had lower motility (i.e., lower percentage of motile cells, percentage of cells moving forward, and hyperactivated cells). These data indicated that motility defects were the most likely cause of the failure of spermatozoa to penetrate the zona pellucida. Cabyr-KO spermatozoa had disorganization of the fibrous sheath and the principal piece axonemal structure in both the distal and proximal regions but no abnormalities in the midpiece. In addition, results suggest that CABYR is not absolutely necessary for PKA substrate phosphorylation and subsequent phosphorylation of tyrosine residues. (Learn more here.)
Publications
Li YF, He W, Kim YH, Mandal A, Digilio L, Klotz K, Flickinger CJ, Herr JC. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath. Reprod Biol Endocrinol. 2010 Aug 23;8:101. PMID: 20731842
Li YF, He W, Mandal A, Kim YH, Digilio L, Klotz K, Flickinger CJ, Herr JC, Herr JC. CABYR binds to AKAP3 and Ropporin in the human sperm fibrous sheath. Asian J Androl. 2011 Mar; 13(2): 266–274. PMID: 21240291
Naaby-Hansen S. Functional and immunological analysis of the human sperm proteome. Dan Med J. 2012 Apr;59(4):B4414. PMID: 22459723
This protein is critical in sperm tail development and, therefore, sperm motility; inhibiting its function could prevent sperm from swimming making men infertile.
The Science
CABYR is a protein in the fibrous sheath of the principal piece of the flagellum and is phosphorylated during capacitation. It also appears to be involved in the development of the fibrous sheath during spermiogenesis, and possibly signal transduction and regulation of flagellar energy supply and movements. It also may function as a regulator of both motility- and head- associated functions such as capacitation and the acrosome reaction. Isoform 1 binds calcium in vitro; Isoform 2 and isoform 6 probably bind calcium; Isoform 3 and isoform 5 do not bind calcium in vitro; Isoform 4 probably does not bind calcium. In vitro assays suggest Cabyr-KO spermatozoa cannot penetrate the zona pellucida, but sperm-oolemma interaction isn’t impaired; Cabyr-KO spermatozoa underwent the acrosome reaction, could bind to the zona pellucida (ZP), and could fuse with the oolemma in ZP-free eggs, but they showed a significant loss in the ability to fertilize cumulus-intact and cumulus-free oocytes. Cabyr-KO spermatozoa had lower motility (i.e., lower percentage of motile cells, percentage of cells moving forward, and hyperactivated cells). These data indicated that motility defects were the most likely cause of the failure of spermatozoa to penetrate the zona pellucida. Cabyr-KO spermatozoa had disorganization of the fibrous sheath and the principal piece axonemal structure in both the distal and proximal regions but no abnormalities in the midpiece. In addition, results suggest that CABYR is not absolutely necessary for PKA substrate phosphorylation and subsequent phosphorylation of tyrosine residues. (Learn more here.)
Publications
Li YF, He W, Kim YH, Mandal A, Digilio L, Klotz K, Flickinger CJ, Herr JC. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath. Reprod Biol Endocrinol. 2010 Aug 23;8:101. PMID: 20731842
Li YF, He W, Mandal A, Kim YH, Digilio L, Klotz K, Flickinger CJ, Herr JC, Herr JC. CABYR binds to AKAP3 and Ropporin in the human sperm fibrous sheath. Asian J Androl. 2011 Mar; 13(2): 266–274. PMID: 21240291
Naaby-Hansen S. Functional and immunological analysis of the human sperm proteome. Dan Med J. 2012 Apr;59(4):B4414. PMID: 22459723
CCNYL1 (Cyclin Y like 1)
Summary
This protein could play a key role in regulating the development of new sperm (spermatogenesis), meaning that preventing its function could render men infertile.
The Science
CCNYL1 is a newly identified member of the cyclin family that is highly expressed in the testis. Cyclins are involved in the regulation of the cell cycle and transcription, however, the function of CCNYL1 is not clearly known. Studies have found that the protein, but not the mRNA, level of cyclin- dependent kinase 16 (CDK16) was decreased in the testis of Ccnyl1-/- mice. Further study demonstrated that CCNYL1 interacted with CDK16 and this interaction mutually increased the stability of these two proteins. Moreover, the interaction increased the kinase activity of CDK16. In addition, an alteration of phosphorylation levels of CDK16 was observed in the presence of CCNYL1. Additionally, studies identified the phosphorylation sites of CDK16 by mass spectrometry and revealed that several phosphorylation modifications on the N-terminal region of CDK16 were indispensable for the CCNYL1 binding and the modulation of CDK16 kinase activity. These results reveal a previously unrecognized role of CCNYL1 in regulating spermatogenesis through the interaction and modulation of CDK16. It is speculated that the interaction of CCNYL1 and CDK16 probably changes the structure or special layout of both proteins, and consequently impairs the ubiquitination process required for the proteasome degradation. As found for other members of the CDKs, CDK16 requires binding of the regulatory partner, CCNYL1, to display its kinase activity. Previous investigations have demonstrated that phosphorylation of CDKs is essential for the binding of cyclin partners and activating their own protein kinase activity, and it has been demonstrated that the phosphorylation of N-terminal of CDK16 was not only critical for the binding of CCNYL1, but also essential for the regulation of CDK16 kinase activity. Of these sites, the S146 and S153 were more critical for the binding of CCNYL1 and for modulating CDK16 kinase activity.
Publications
Zi, Z., Zhang, Z., Li, Q., An, W., Zeng, L., Gao, D., . . . Wu, J. (2015). CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse. PLOS Genetics,11(8).
Zeng L, Cai C, Li S, Wang W, Li Y, Chen J, Zhu X, Zeng YA. Essential Roles of Cyclin Y-Like 1 and Cyclin Y in Dividing Wnt-Responsive Mammary Stem/Progenitor Cells. PLoS Genet. 2016 May 20;12(5):e1006055. doi: 10.1371/journal.pgen.1006055. PMID: 27203244; PMCID: PMC4874687.
Zhang X, Zhou W, Zhang P, Gao F, Zhao X, Shum WW, Zeng X. Cabs1 Maintains Structural Integrity of Mouse Sperm Flagella during Epididymal Transit of Sperm. Int J Mol Sci. 2021 Jan 11;22(2):652. doi: 10.3390/ijms22020652. PMID: 33440775; PMCID: PMC7827751.
This protein could play a key role in regulating the development of new sperm (spermatogenesis), meaning that preventing its function could render men infertile.
The Science
CCNYL1 is a newly identified member of the cyclin family that is highly expressed in the testis. Cyclins are involved in the regulation of the cell cycle and transcription, however, the function of CCNYL1 is not clearly known. Studies have found that the protein, but not the mRNA, level of cyclin- dependent kinase 16 (CDK16) was decreased in the testis of Ccnyl1-/- mice. Further study demonstrated that CCNYL1 interacted with CDK16 and this interaction mutually increased the stability of these two proteins. Moreover, the interaction increased the kinase activity of CDK16. In addition, an alteration of phosphorylation levels of CDK16 was observed in the presence of CCNYL1. Additionally, studies identified the phosphorylation sites of CDK16 by mass spectrometry and revealed that several phosphorylation modifications on the N-terminal region of CDK16 were indispensable for the CCNYL1 binding and the modulation of CDK16 kinase activity. These results reveal a previously unrecognized role of CCNYL1 in regulating spermatogenesis through the interaction and modulation of CDK16. It is speculated that the interaction of CCNYL1 and CDK16 probably changes the structure or special layout of both proteins, and consequently impairs the ubiquitination process required for the proteasome degradation. As found for other members of the CDKs, CDK16 requires binding of the regulatory partner, CCNYL1, to display its kinase activity. Previous investigations have demonstrated that phosphorylation of CDKs is essential for the binding of cyclin partners and activating their own protein kinase activity, and it has been demonstrated that the phosphorylation of N-terminal of CDK16 was not only critical for the binding of CCNYL1, but also essential for the regulation of CDK16 kinase activity. Of these sites, the S146 and S153 were more critical for the binding of CCNYL1 and for modulating CDK16 kinase activity.
Publications
Zi, Z., Zhang, Z., Li, Q., An, W., Zeng, L., Gao, D., . . . Wu, J. (2015). CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse. PLOS Genetics,11(8).
Zeng L, Cai C, Li S, Wang W, Li Y, Chen J, Zhu X, Zeng YA. Essential Roles of Cyclin Y-Like 1 and Cyclin Y in Dividing Wnt-Responsive Mammary Stem/Progenitor Cells. PLoS Genet. 2016 May 20;12(5):e1006055. doi: 10.1371/journal.pgen.1006055. PMID: 27203244; PMCID: PMC4874687.
Zhang X, Zhou W, Zhang P, Gao F, Zhao X, Shum WW, Zeng X. Cabs1 Maintains Structural Integrity of Mouse Sperm Flagella during Epididymal Transit of Sperm. Int J Mol Sci. 2021 Jan 11;22(2):652. doi: 10.3390/ijms22020652. PMID: 33440775; PMCID: PMC7827751.
CCR6 (C-C Motif Chemokine Receptor 6)
Summary
This protein may be critical in facilitating both sperm motility as well as the ability for sperm to locate the egg whilst in the female reproductive tract; impeding its functioning could effectively render sperm incapable of swimming and locating an egg to fertilize.
The Science
CCR6 is a receptor common to several chemoattractant peptides. Before fertilization, sperm must receive and process environmental signals to locate the oocyte. These chemoattractant molecules originate from the oocyte, its surrounding investments, and are also found in high concentrations in seminal plasma. The interactions of the sperm with these chemoattractants primarily influence sperm movement, directing the sperm to the site of fertilization. The chemokine receptor CCR6 was found in sperm primarily localized to the principal piece of the sperm tail, which is involved in sperm motility and hyperactivation. The binding molecules of CCR6 include CCL20 and mouse DEFB29 (human DEFB116). While CCL20 is found in human seminal plasma, endometrial fluid and follicular fluid, DFB29 is highly expressed in the cauda epididymis. Capacitated sperm demonstrated chemotactic response to recombinant CCL20 and follicular fluid with different intensities. CCR6 is a G-protein coupled receptor, which upon binding to their cognate chemokine ligand undergoes conformational changes giving rise to activation of intracellular effectors and signal transduction pathways that result in various cellular responses (e.g., cell migration, leukocyte degranulation, cell differentiation, angiogenesis or angiostasis). Human DEFB1 interacts with CCR6 in sperm and triggers Ca2+ mobilization, which is important for sperm motility. Interference with CCR6 function also reduces motility and bactericidal activity of normal sperm. CCR6 is co-localized with CatSper and is suggested to play a critical role in ligand-induced CatSper activation resulting in sperm hyperactivation.
Publications
Bennett, L. D., Fox, J. M., & Signoret, N. (2011). Mechanisms regulating chemokine receptor activity. Immunology,134(3), 246-256.
Caballero-Campo P, Buffone MG, Benencia F, Conejo-García JR, Rinaudo PF, Gerton GL. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J Cell Physiol. 2014 Jan;229(1):68-78. doi: 10.1002/jcp.24418. PMID: 23765988; PMCID: PMC3807638.
Diao R, Wang T, Fok KL, Li X, Ruan Y, Yu MK, Cheng Y, Chen Y, Chen H, Mou L, Cai X, Wang Y, Cai Z, Zeng X, Chan HC. CCR6 is required for ligand-induced CatSper activation in human sperm. Oncotarget. 2017 Sep 5;8(53):91445-91458. doi: 10.18632/oncotarget.20651. PMID: 29207656; PMCID: PMC5710936.
This protein may be critical in facilitating both sperm motility as well as the ability for sperm to locate the egg whilst in the female reproductive tract; impeding its functioning could effectively render sperm incapable of swimming and locating an egg to fertilize.
The Science
CCR6 is a receptor common to several chemoattractant peptides. Before fertilization, sperm must receive and process environmental signals to locate the oocyte. These chemoattractant molecules originate from the oocyte, its surrounding investments, and are also found in high concentrations in seminal plasma. The interactions of the sperm with these chemoattractants primarily influence sperm movement, directing the sperm to the site of fertilization. The chemokine receptor CCR6 was found in sperm primarily localized to the principal piece of the sperm tail, which is involved in sperm motility and hyperactivation. The binding molecules of CCR6 include CCL20 and mouse DEFB29 (human DEFB116). While CCL20 is found in human seminal plasma, endometrial fluid and follicular fluid, DFB29 is highly expressed in the cauda epididymis. Capacitated sperm demonstrated chemotactic response to recombinant CCL20 and follicular fluid with different intensities. CCR6 is a G-protein coupled receptor, which upon binding to their cognate chemokine ligand undergoes conformational changes giving rise to activation of intracellular effectors and signal transduction pathways that result in various cellular responses (e.g., cell migration, leukocyte degranulation, cell differentiation, angiogenesis or angiostasis). Human DEFB1 interacts with CCR6 in sperm and triggers Ca2+ mobilization, which is important for sperm motility. Interference with CCR6 function also reduces motility and bactericidal activity of normal sperm. CCR6 is co-localized with CatSper and is suggested to play a critical role in ligand-induced CatSper activation resulting in sperm hyperactivation.
Publications
Bennett, L. D., Fox, J. M., & Signoret, N. (2011). Mechanisms regulating chemokine receptor activity. Immunology,134(3), 246-256.
Caballero-Campo P, Buffone MG, Benencia F, Conejo-García JR, Rinaudo PF, Gerton GL. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J Cell Physiol. 2014 Jan;229(1):68-78. doi: 10.1002/jcp.24418. PMID: 23765988; PMCID: PMC3807638.
Diao R, Wang T, Fok KL, Li X, Ruan Y, Yu MK, Cheng Y, Chen Y, Chen H, Mou L, Cai X, Wang Y, Cai Z, Zeng X, Chan HC. CCR6 is required for ligand-induced CatSper activation in human sperm. Oncotarget. 2017 Sep 5;8(53):91445-91458. doi: 10.18632/oncotarget.20651. PMID: 29207656; PMCID: PMC5710936.
CDK2 (Cyclin-dependent Kinase 2)
Summary
Essential for mitotic cell division, male and female animal models show infertility when this gene is disrupted.
The Science
CDK2 is the catalytic subunit of the cyclin-dependent protein kinase complex, which regulates progression through the cell cycle. Activity of this protein is especially critical during the G1 to S phase transition. Males display normal proliferation of spermatogonia through day 30 when seminiferous tubules are almost completely depleted of germ cells, by day 120, testes are atrophic (20% normal size), normal levels of spermatogonia, Sertoli and Leydig cells, few primary spermatocytes, no post meiotic cells. Abnormal meiosis is shown due to a failure of chromosome pairing, and meiosis fails to proceed past the pachytene stage of prophase I. (Learn more here.)
Publications
Li, Y., Zhang, J., Gao, W., Zhang, L., Pan, Y., Zhang, S., & Wang, Y. (2015). Insights on Structural Characteristics and Ligand Binding Mechanisms of CDK2. International Journal of Molecular Sciences,16(12), 9314-9340.
Ortega, S., Prieto, I., Odajima, J., Martín, A., Dubus, P., Sotillo, R., . . . Barbacid, M. (2003). Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nature Genetics,35(1), 25-31.
Singh, P., & Schimenti, J. C. (2015). The genetics of human infertility by functional interrogation of SNPs in mice. Proceedings of the National Academy of Sciences,112(33), 10431-10436.
Essential for mitotic cell division, male and female animal models show infertility when this gene is disrupted.
The Science
CDK2 is the catalytic subunit of the cyclin-dependent protein kinase complex, which regulates progression through the cell cycle. Activity of this protein is especially critical during the G1 to S phase transition. Males display normal proliferation of spermatogonia through day 30 when seminiferous tubules are almost completely depleted of germ cells, by day 120, testes are atrophic (20% normal size), normal levels of spermatogonia, Sertoli and Leydig cells, few primary spermatocytes, no post meiotic cells. Abnormal meiosis is shown due to a failure of chromosome pairing, and meiosis fails to proceed past the pachytene stage of prophase I. (Learn more here.)
Publications
Li, Y., Zhang, J., Gao, W., Zhang, L., Pan, Y., Zhang, S., & Wang, Y. (2015). Insights on Structural Characteristics and Ligand Binding Mechanisms of CDK2. International Journal of Molecular Sciences,16(12), 9314-9340.
Ortega, S., Prieto, I., Odajima, J., Martín, A., Dubus, P., Sotillo, R., . . . Barbacid, M. (2003). Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nature Genetics,35(1), 25-31.
Singh, P., & Schimenti, J. C. (2015). The genetics of human infertility by functional interrogation of SNPs in mice. Proceedings of the National Academy of Sciences,112(33), 10431-10436.
CETN1 (Centrin 1)
Summary
This protein appears to play a key role in late-stage sperm development. Restricting its functioning could render men infertile.
The Science
CETN1 is a calcium-binding protein that represents an important component of the sperm centriole, a key structure during fertilization responsible for the creation of a well- organized sperm aster in the zygote. In human sperm showing both dysplasia of the fibrous sheath and alterations in the head-mid-piece junction, CETN1 is present but its localization is abnormal and is immobile. When introduced to oocytes through ICSI, these sperm demonstrate low fertility potential. Significantly lower levels of CETN1 observed in oligoasthenozoospermic human sperm suggests a role for CETN1 in motility. Since CETN1 is a calcium binding protein, it is speculated to be involved in calcium-induced uncoupling of the axoneme from the basal body during fertilization. Light and electron microscopy analyses of Cetn1-/- spermatids revealed failures in centriole rearrangement during basal body maturation and in the basal-body–nucleus connection. These results confirm an essential role for CETN1 in late steps of spermiogenesis and spermatid maturation.
Publications
Avasthi, P., Scheel, J. F., Ying, G., Frederick, J. M., Baehr, W., & Wolfrum, U. (2013). Germline deletion of Cetn1 causes infertility in male mice. Journal of Cell Science,126(14), 3204-3213.
Hinduja, I, Zaveri, K, Baliga, N. (2008). Human sperm centrin levels & outcome of intracytoplasmic sperm injection (ICSI)--a pilot study. Indian J. Med. Res, 128(5):606-10.
Hinduja, I., Baliga, N., & Zaveri, K. (2010). Correlation of human sperm centrosomal proteins with fertility. Journal of Human Reproductive Sciences,3(2), 95.
This protein appears to play a key role in late-stage sperm development. Restricting its functioning could render men infertile.
The Science
CETN1 is a calcium-binding protein that represents an important component of the sperm centriole, a key structure during fertilization responsible for the creation of a well- organized sperm aster in the zygote. In human sperm showing both dysplasia of the fibrous sheath and alterations in the head-mid-piece junction, CETN1 is present but its localization is abnormal and is immobile. When introduced to oocytes through ICSI, these sperm demonstrate low fertility potential. Significantly lower levels of CETN1 observed in oligoasthenozoospermic human sperm suggests a role for CETN1 in motility. Since CETN1 is a calcium binding protein, it is speculated to be involved in calcium-induced uncoupling of the axoneme from the basal body during fertilization. Light and electron microscopy analyses of Cetn1-/- spermatids revealed failures in centriole rearrangement during basal body maturation and in the basal-body–nucleus connection. These results confirm an essential role for CETN1 in late steps of spermiogenesis and spermatid maturation.
Publications
Avasthi, P., Scheel, J. F., Ying, G., Frederick, J. M., Baehr, W., & Wolfrum, U. (2013). Germline deletion of Cetn1 causes infertility in male mice. Journal of Cell Science,126(14), 3204-3213.
Hinduja, I, Zaveri, K, Baliga, N. (2008). Human sperm centrin levels & outcome of intracytoplasmic sperm injection (ICSI)--a pilot study. Indian J. Med. Res, 128(5):606-10.
Hinduja, I., Baliga, N., & Zaveri, K. (2010). Correlation of human sperm centrosomal proteins with fertility. Journal of Human Reproductive Sciences,3(2), 95.
CKS2 (CDC28 Protein Kinase Regulatory Subunit 2)
Summary
This protein has been shown to be critical in the initial stages of cell division, including the development of sperm cells; impeding it could prevent the development of sperm cells.
The Science
The mammalian cyclin kinase subunit (CKS) family has two members; cyclin-dependent kinase subunit 1 (CKS1) and CKS2. Human CKS2 is essential for the first metaphase/anaphase transition of mammalian meiosis. While individually deleting either CSK1 or CSK2 has no impact on viability, simultaneous disruption results in embryonic lethality. In studies, the observed failure of germ cell development in CKS2–/– mice most likely resulted from a lack of CKS1 expression that is necessary to compensate for CKS2 loss of function in the germ line rather than from a germline–specific function of CKS2. To test this hypothesis, a study microinjected prophase-arrested, germinal vesicle (GV) stage CKS2–/– oocytes with either CKS1 or CKS2 mRNA and then allowed to undergo GV breakdown. Both CKS1 and CKS2 mRNA could rescue the metaphase I arrest of CKS2–/– oocytes, consistent with the hypothesis that germ line exclusion of CKS1 expression renders CKS2 essential. However, a mutant form of CKS2, defective in CDK interaction, could not overcome the M1 arrest, which indicates that the required function of CKS2 in oocytes involves interactions with a CDK.
Publications
Martinsson-Ahlzén HS, Liberal V, Grünenfelder B, Chaves SR, Spruck CH, Reed SI. Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol Cell Biol. 2008 Sep;28(18):5698-709. doi: 10.1128/MCB.01833-07. Epub 2008 Jul 14. PMID: 18625720; PMCID: PMC2546922.
Parge, H., Arvai, A., & Tainer, J. (1995). Human Ckshs2 Atomic Structure: A Role For Its Hexameric Assembly In Cell Cycle Control.
Spruck, C. H. (2003). Requirement of Cks2 for the First Metaphase/Anaphase Transition of Mammalian Meiosis. Science,300(5619), 647-650
This protein has been shown to be critical in the initial stages of cell division, including the development of sperm cells; impeding it could prevent the development of sperm cells.
The Science
The mammalian cyclin kinase subunit (CKS) family has two members; cyclin-dependent kinase subunit 1 (CKS1) and CKS2. Human CKS2 is essential for the first metaphase/anaphase transition of mammalian meiosis. While individually deleting either CSK1 or CSK2 has no impact on viability, simultaneous disruption results in embryonic lethality. In studies, the observed failure of germ cell development in CKS2–/– mice most likely resulted from a lack of CKS1 expression that is necessary to compensate for CKS2 loss of function in the germ line rather than from a germline–specific function of CKS2. To test this hypothesis, a study microinjected prophase-arrested, germinal vesicle (GV) stage CKS2–/– oocytes with either CKS1 or CKS2 mRNA and then allowed to undergo GV breakdown. Both CKS1 and CKS2 mRNA could rescue the metaphase I arrest of CKS2–/– oocytes, consistent with the hypothesis that germ line exclusion of CKS1 expression renders CKS2 essential. However, a mutant form of CKS2, defective in CDK interaction, could not overcome the M1 arrest, which indicates that the required function of CKS2 in oocytes involves interactions with a CDK.
Publications
Martinsson-Ahlzén HS, Liberal V, Grünenfelder B, Chaves SR, Spruck CH, Reed SI. Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol Cell Biol. 2008 Sep;28(18):5698-709. doi: 10.1128/MCB.01833-07. Epub 2008 Jul 14. PMID: 18625720; PMCID: PMC2546922.
Parge, H., Arvai, A., & Tainer, J. (1995). Human Ckshs2 Atomic Structure: A Role For Its Hexameric Assembly In Cell Cycle Control.
Spruck, C. H. (2003). Requirement of Cks2 for the First Metaphase/Anaphase Transition of Mammalian Meiosis. Science,300(5619), 647-650
CLGN (Calmegin)
Summary
This molecule is expressed during early stages of sperm cell development, and may be necessary for creating fully functioning sperm.
The Science
Calmegin is a putative testis-specific molecular chaperone which is absent from mature sperm and is probably responsible for the correct folding and transport of testis proteins. Consistent with this idea, calmegin directly associates with fertilin α and β in the testis, where it is involved in their heterodimerization. In addition, the absence of fertilin β in sperm from calmegin knockout animals suggests that calmegin is necessary for the appearance of this protein on the sperm surface. If not targeted correctly during spermatogenesis, proteins other than fertilin β could be missing in sperm from the calmegin-/- animals. CLGN expression is observed during the meiosis phase of spermatogenesis. (Please note: fertilin α and β are aliases used for ADAM 1 and 2, respectively).
Publications
Ikawa, M., Wada, I., Kominami, K. et al. The putative chaperone calmegin is required for sperm fertility. Nature 387, 607–611 (1997). https://doi.org/10.1038/42484
Ikawa M, Nakanishi T, Yamada S, Wada I, Kominami K, Tanaka H, Nozaki M, Nishimune Y, Okabe M. Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility. Dev Biol. 2001 Dec 1;240(1):254-61. doi: 10.1006/dbio.2001.0462. PMID: 11784061.
Ikawa M, Tokuhiro K, Yamaguchi R, Benham AM, Tamura T, Wada I, Satouh Y, Inoue N, Okabe M. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem. 2011 Feb 18;286(7):5639-46. doi: 10.1074/jbc.M110.140152. Epub 2010 Dec 3. PMID: 21131354; PMCID: PMC3037677.
This molecule is expressed during early stages of sperm cell development, and may be necessary for creating fully functioning sperm.
The Science
Calmegin is a putative testis-specific molecular chaperone which is absent from mature sperm and is probably responsible for the correct folding and transport of testis proteins. Consistent with this idea, calmegin directly associates with fertilin α and β in the testis, where it is involved in their heterodimerization. In addition, the absence of fertilin β in sperm from calmegin knockout animals suggests that calmegin is necessary for the appearance of this protein on the sperm surface. If not targeted correctly during spermatogenesis, proteins other than fertilin β could be missing in sperm from the calmegin-/- animals. CLGN expression is observed during the meiosis phase of spermatogenesis. (Please note: fertilin α and β are aliases used for ADAM 1 and 2, respectively).
Publications
Ikawa, M., Wada, I., Kominami, K. et al. The putative chaperone calmegin is required for sperm fertility. Nature 387, 607–611 (1997). https://doi.org/10.1038/42484
Ikawa M, Nakanishi T, Yamada S, Wada I, Kominami K, Tanaka H, Nozaki M, Nishimune Y, Okabe M. Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility. Dev Biol. 2001 Dec 1;240(1):254-61. doi: 10.1006/dbio.2001.0462. PMID: 11784061.
Ikawa M, Tokuhiro K, Yamaguchi R, Benham AM, Tamura T, Wada I, Satouh Y, Inoue N, Okabe M. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem. 2011 Feb 18;286(7):5639-46. doi: 10.1074/jbc.M110.140152. Epub 2010 Dec 3. PMID: 21131354; PMCID: PMC3037677.
CNR1/2 (Cannabinoid Receptor 1 & 2)
Summary
These protein receptors are responsible for key roles in sperm motility and sperm’s ability to fertilize an egg.
The Science
CNR1 and 2 are members of the G-protein coupled receptors that bind endogenous cannabinoids, principally anadamide (AEA) and 2- arachidonoylethanol amine (2-AG). Marijuana/cannabis also bind these receptors. The control of sperm motility, as well as the induction of the acrosome reaction (AR), has been ascribed to CB1 activation although more recently also a role for CB2 has been documented. The presence of CB(1) and CB(2) receptors has been verified in human spermatozoa, and the distribution of both of these receptors was distinct. Incubation with selective cannabinoid receptor agonists induced a significant reduction in the proportion of rapidly progressive motile spermatozoa, and whereas the CB(1) agonist increased the proportion of immobile sperm cells, the CB(2) receptor agonist increased the slow/sluggish progressive sperm cell population. The effect of the CB(2) agonist was antagonized by the CB(2)-specific antagonist.
Publications
Agirregoitia E, Carracedo A, Subirán N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, Irazusta J. The CB(2) cannabinoid receptor regulates human sperm cell motility. Fertil Steril. 2010 Mar 15;93(5):1378-87. doi: 10.1016/j.fertnstert.2009.01.153. Epub 2009 Mar 27. PMID: 19328464.
Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011 Jan 1;16:498-516. doi: 10.2741/3701. PMID: 21196184.
Lewis SE, Maccarrone M. Endocannabinoids, sperm biology and human fertility. Pharmacol Res. 2009 Aug;60(2):126-31. doi: 10.1016/j.phrs.2009.02.009. Epub 2009 Mar 4. PMID: 19559363.
These protein receptors are responsible for key roles in sperm motility and sperm’s ability to fertilize an egg.
The Science
CNR1 and 2 are members of the G-protein coupled receptors that bind endogenous cannabinoids, principally anadamide (AEA) and 2- arachidonoylethanol amine (2-AG). Marijuana/cannabis also bind these receptors. The control of sperm motility, as well as the induction of the acrosome reaction (AR), has been ascribed to CB1 activation although more recently also a role for CB2 has been documented. The presence of CB(1) and CB(2) receptors has been verified in human spermatozoa, and the distribution of both of these receptors was distinct. Incubation with selective cannabinoid receptor agonists induced a significant reduction in the proportion of rapidly progressive motile spermatozoa, and whereas the CB(1) agonist increased the proportion of immobile sperm cells, the CB(2) receptor agonist increased the slow/sluggish progressive sperm cell population. The effect of the CB(2) agonist was antagonized by the CB(2)-specific antagonist.
Publications
Agirregoitia E, Carracedo A, Subirán N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, Irazusta J. The CB(2) cannabinoid receptor regulates human sperm cell motility. Fertil Steril. 2010 Mar 15;93(5):1378-87. doi: 10.1016/j.fertnstert.2009.01.153. Epub 2009 Mar 27. PMID: 19328464.
Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011 Jan 1;16:498-516. doi: 10.2741/3701. PMID: 21196184.
Lewis SE, Maccarrone M. Endocannabinoids, sperm biology and human fertility. Pharmacol Res. 2009 Aug;60(2):126-31. doi: 10.1016/j.phrs.2009.02.009. Epub 2009 Mar 4. PMID: 19559363.
DEFB106A/B (Defensin beta 106A & B)
Summary
This family of peptides are found in the reproductive tract and could drastically impact sperm tail development and, therefore, motility.
The Science
Defensins form a family of antimicrobial and cytotoxic peptides made by neutrophils. β-antimicrobials are important in the first defense response to invading organisms. They are predominantly produced at surfaces in contact with the outside environment and these include the skin, airway, and reproductive tract. Studies have shown that when a subset of nine β-defensin genes are deleted from mice, the main consequence is that the male mice are completely infertile. When normal sperm leave the male and enter the female reproductive tract they are triggered to undergo a reaction that alters the membrane properties of the sperm and allows fertilization. It has been shown that sperm isolated from the male mice, that no longer make these β- defensins, are prematurely ready to fertilize an egg. It is far too early for this to happen and as a consequence the sperm are severely reduced in their ability to move and have a major defect in the structure of their tail. Evidence has been provided that the reason this has happened is due to a dysregulation of calcium transport. This is important for understanding defensin gene function in a living organism, and may enable the design of novel contraceptives with additional antibiotic ability.
Publications
Falanga A, Nigro E, De Biasi MG, Daniele A, Morelli G, Galdiero S, Scudiero O. Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics. Molecules. 2017 Jul 20;22(7):1217. doi: 10.3390/molecules22071217. PMID: 28726740; PMCID: PMC6152268.
Semple F, Dorin JR. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4(4):337-48. doi: 10.1159/000336619. Epub 2012 Mar 21. PMID: 22441423; PMCID: PMC6784047.
Zhao Y, Diao H, Ni Z, Hu S, Yu H, Zhang Y. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell Mol Life Sci. 2011 Feb;68(4):697-708. doi: 10.1007/s00018-010-0478-4. Epub 2010 Aug 8. PMID: 20694738.
This family of peptides are found in the reproductive tract and could drastically impact sperm tail development and, therefore, motility.
The Science
Defensins form a family of antimicrobial and cytotoxic peptides made by neutrophils. β-antimicrobials are important in the first defense response to invading organisms. They are predominantly produced at surfaces in contact with the outside environment and these include the skin, airway, and reproductive tract. Studies have shown that when a subset of nine β-defensin genes are deleted from mice, the main consequence is that the male mice are completely infertile. When normal sperm leave the male and enter the female reproductive tract they are triggered to undergo a reaction that alters the membrane properties of the sperm and allows fertilization. It has been shown that sperm isolated from the male mice, that no longer make these β- defensins, are prematurely ready to fertilize an egg. It is far too early for this to happen and as a consequence the sperm are severely reduced in their ability to move and have a major defect in the structure of their tail. Evidence has been provided that the reason this has happened is due to a dysregulation of calcium transport. This is important for understanding defensin gene function in a living organism, and may enable the design of novel contraceptives with additional antibiotic ability.
Publications
Falanga A, Nigro E, De Biasi MG, Daniele A, Morelli G, Galdiero S, Scudiero O. Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics. Molecules. 2017 Jul 20;22(7):1217. doi: 10.3390/molecules22071217. PMID: 28726740; PMCID: PMC6152268.
Semple F, Dorin JR. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4(4):337-48. doi: 10.1159/000336619. Epub 2012 Mar 21. PMID: 22441423; PMCID: PMC6784047.
Zhao Y, Diao H, Ni Z, Hu S, Yu H, Zhang Y. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell Mol Life Sci. 2011 Feb;68(4):697-708. doi: 10.1007/s00018-010-0478-4. Epub 2010 Aug 8. PMID: 20694738.
DNAH17 (Dynein axonemal heavy chain 17)
Summary
This gene is associated with spermatogenesis as well multiple morphological abnormalities of sperm flagella, and has been shown to be related with infertility in men.
The Science
Dynein axonemal heavy chain 17 (DNAH17) is a microtubule-based molecular motor possessing ATPase activity that can convert the chemical energy of ATP into relative sliding between adjacent microtubule doublets to generate ciliary bending, playing a central role in ciliary beats and sperm motility. Dnah17 knockout rats were generated by CRISPR/Cas9 gene editing, and homozygous male rats were noted to be infertile, with significantly decreased number of sperm, suggesting cryptozoospermia that was further confirmed by histologic studies. DNAH mutant sperm are incompetent for intracytoplasmic sperm injection and show decreased DNAH8 expression.
Publications
Whitfield M, Thomas L, Bequignon E, Schmitt A, Stouvenel L, Montantin G, Tissier S, Duquesnoy P, Copin B, Chantot S, Dastot F, Faucon C, Barbotin AL, Loyens A, Siffroi JP, Papon JF, Escudier E, Amselem S, Mitchell V, Touré A, Legendre M. Mutations in DNAH17, Encoding a Sperm-Specific Axonemal Outer Dynein Arm Heavy Chain, Cause Isolated Male Infertility Due to Asthenozoospermia. Am J Hum Genet. 2019 Jul 3;105(1):198-212. doi: 10.1016/j.ajhg.2019.04.015. Epub 2019 Jun 6. PMID: 31178125; PMCID: PMC6612517.
Zheng R, Sun Y, Jiang C, Chen D, Yang Y, Shen Y. A novel mutation in DNAH17 is present in a patient with multiple morphological abnormalities of the flagella. Reprod Biomed Online. 2021 Sep;43(3):532-541. doi: 10.1016/j.rbmo.2021.05.009. Epub 2021 May 21. PMID: 34373205.
Chen L, Ouyang J, Li X, Xiao X, Sun W, Li S, Zhou L, Liao Y, Zhang Q. DNAH17 is essential for rat spermatogenesis and fertility. J Genet. 2021;100:14. PMID: 33764336.
This gene is associated with spermatogenesis as well multiple morphological abnormalities of sperm flagella, and has been shown to be related with infertility in men.
The Science
Dynein axonemal heavy chain 17 (DNAH17) is a microtubule-based molecular motor possessing ATPase activity that can convert the chemical energy of ATP into relative sliding between adjacent microtubule doublets to generate ciliary bending, playing a central role in ciliary beats and sperm motility. Dnah17 knockout rats were generated by CRISPR/Cas9 gene editing, and homozygous male rats were noted to be infertile, with significantly decreased number of sperm, suggesting cryptozoospermia that was further confirmed by histologic studies. DNAH mutant sperm are incompetent for intracytoplasmic sperm injection and show decreased DNAH8 expression.
Publications
Whitfield M, Thomas L, Bequignon E, Schmitt A, Stouvenel L, Montantin G, Tissier S, Duquesnoy P, Copin B, Chantot S, Dastot F, Faucon C, Barbotin AL, Loyens A, Siffroi JP, Papon JF, Escudier E, Amselem S, Mitchell V, Touré A, Legendre M. Mutations in DNAH17, Encoding a Sperm-Specific Axonemal Outer Dynein Arm Heavy Chain, Cause Isolated Male Infertility Due to Asthenozoospermia. Am J Hum Genet. 2019 Jul 3;105(1):198-212. doi: 10.1016/j.ajhg.2019.04.015. Epub 2019 Jun 6. PMID: 31178125; PMCID: PMC6612517.
Zheng R, Sun Y, Jiang C, Chen D, Yang Y, Shen Y. A novel mutation in DNAH17 is present in a patient with multiple morphological abnormalities of the flagella. Reprod Biomed Online. 2021 Sep;43(3):532-541. doi: 10.1016/j.rbmo.2021.05.009. Epub 2021 May 21. PMID: 34373205.
Chen L, Ouyang J, Li X, Xiao X, Sun W, Li S, Zhou L, Liao Y, Zhang Q. DNAH17 is essential for rat spermatogenesis and fertility. J Genet. 2021;100:14. PMID: 33764336.
FSCB (Fibrous Sheath CABYR Binding Protein)
Summary
This protein is believed to be critical in the development of sperm tails and, therefore, sperm motility; inhibiting them could render men infertile due to sperm’s inability to swim properly.
The Science
FSCB is involved in spermatozoa capacitation and may be involved in the later stages of biogenesis of the sperm fibrous sheath. It inhibits ROPN1 and ROPN1L SUMOylation and binds calcium. FSCB is a post-meiotic protein first expressed at step 11 of mouse spermatogenesis in the elongating spermatids, and it subsequently incorporates into the flagellar principal piece of the sperm. Ultrastructurally, FSCB localized to a cortical layer of intermediate electron density at the surface of the ribs and longitudinal columns of the fibrous sheath. Due to its temporal appearance during spermiogenesis and location at the cortex of the fibrous sheath, FSCB is postulated to be involved in the later stages of fibrous sheath assembly.
Publications
Kaneto M, Krisfalusi M, Eddy EM, O'Brien DA, Miki K. Bicarbonate-induced phosphorylation of p270 protein in mouse sperm by cAMP-dependent protein kinase. Mol Reprod Dev. 2008 Jun;75(6):1045-53. doi: 10.1002/mrd.20839. PMID: 18357561.
Fiedler SE, Dudiki T, Vijayaraghavan S, Carr DW. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol Reprod. 2013 Feb 21;88(2):41. doi: 10.1095/biolreprod.112.105262. PMID: 23303679; PMCID: PMC4434999.
Zhang X, Chen M, Yu R, Liu B, Tian Z, Liu S. FSCB phosphorylation regulates mouse spermatozoa capacitation through suppressing SUMOylation of ROPN1/ROPN1L. Am J Transl Res. 2016;8(6):2776-2782. Published 2016 Jun 15.
This protein is believed to be critical in the development of sperm tails and, therefore, sperm motility; inhibiting them could render men infertile due to sperm’s inability to swim properly.
The Science
FSCB is involved in spermatozoa capacitation and may be involved in the later stages of biogenesis of the sperm fibrous sheath. It inhibits ROPN1 and ROPN1L SUMOylation and binds calcium. FSCB is a post-meiotic protein first expressed at step 11 of mouse spermatogenesis in the elongating spermatids, and it subsequently incorporates into the flagellar principal piece of the sperm. Ultrastructurally, FSCB localized to a cortical layer of intermediate electron density at the surface of the ribs and longitudinal columns of the fibrous sheath. Due to its temporal appearance during spermiogenesis and location at the cortex of the fibrous sheath, FSCB is postulated to be involved in the later stages of fibrous sheath assembly.
Publications
Kaneto M, Krisfalusi M, Eddy EM, O'Brien DA, Miki K. Bicarbonate-induced phosphorylation of p270 protein in mouse sperm by cAMP-dependent protein kinase. Mol Reprod Dev. 2008 Jun;75(6):1045-53. doi: 10.1002/mrd.20839. PMID: 18357561.
Fiedler SE, Dudiki T, Vijayaraghavan S, Carr DW. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol Reprod. 2013 Feb 21;88(2):41. doi: 10.1095/biolreprod.112.105262. PMID: 23303679; PMCID: PMC4434999.
Zhang X, Chen M, Yu R, Liu B, Tian Z, Liu S. FSCB phosphorylation regulates mouse spermatozoa capacitation through suppressing SUMOylation of ROPN1/ROPN1L. Am J Transl Res. 2016;8(6):2776-2782. Published 2016 Jun 15.
KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1)
Summary
This protein appears to play a key role in facilitating the development of new sperm (spermatogenesis).
The Science
KHDRBS1 (previously known as Sam68) belongs to the evolutionarily conserved signal transduction activator of RNA (STAR) family of RNA-binding proteins (RBPs), which play key roles during cell differentiation and development. KHDRBS1 was originally described as a scaffold protein recruited in various signal transduction pathways. However, KHDRBS1 also modulates several steps of RNA metabolism such as alternative splicing, nuclear export and cytoplasmic utilization or translation of viral and cellular mRNAs. Devoid of an enzymatic activity, KHDRBS1 functions as an adaptor molecule mediating numerous protein- and RNA-interactions. Male KHDRBS1 knockout mice are infertile due to aberrant differentiation of round spermatids into mature spermatozoa. A subset of testicular transcripts that are affected by KHDRBS1 ablation and found an enrichment in mRNAs encoding proteins involved in cell proliferation and survival has been identified. Several of these mRNAs are bound by KHDRBS1 in germ cells. Moreover, studies have provided evidence that, upon meiotic divisions, KHDRBS1 associates with the translation initiation complex and regulates polysomal loading and translation of the mRNAs encoding SPAG16, a cytoskeletal protein required for sperm motility and fertility; NEDD1, a centrosomal protein required for microtubule organization; and SPDYA, a cell cycle regulator. Findings suggest that KHDRBS1 loss of function leads to male infertility by restricting translation of a selected group of mRNA transcripts.
Publications
Asbach B, Ludwig C, Saksela K, Wagner R. Comprehensive analysis of interactions between the Src-associated protein in mitosis of 68 kDa and the human Src-homology 3 proteome. PLoS One. 2012;7(6):e38540. doi: 10.1371/journal.pone.0038540. Epub 2012 Jun 20. PMID: 22745667; PMCID: PMC3379994.
Enrica Bianchi, Federica Barbagallo, Claudia Valeri, Raffaele Geremia, Antonietta Salustri, Massimo De Felici, Claudio Sette, Ablation of the Sam68 gene impairs female fertility and gonadotropin-dependent follicle development, Human Molecular Genetics, Volume 19, Issue 24, 15 December 2010, Pages 4886–4894, https://doi.org/10.1093/hmg/ddq422
Paronetto MP, Messina V, Bianchi E, Barchi M, Vogel G, Moretti C, Palombi F, Stefanini M, Geremia R, Richard S, Sette C. Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J Cell Biol. 2009 Apr 20;185(2):235-49. doi: 10.1083/jcb.200811138. PMID: 19380878; PMCID: PMC2700383.
This protein appears to play a key role in facilitating the development of new sperm (spermatogenesis).
The Science
KHDRBS1 (previously known as Sam68) belongs to the evolutionarily conserved signal transduction activator of RNA (STAR) family of RNA-binding proteins (RBPs), which play key roles during cell differentiation and development. KHDRBS1 was originally described as a scaffold protein recruited in various signal transduction pathways. However, KHDRBS1 also modulates several steps of RNA metabolism such as alternative splicing, nuclear export and cytoplasmic utilization or translation of viral and cellular mRNAs. Devoid of an enzymatic activity, KHDRBS1 functions as an adaptor molecule mediating numerous protein- and RNA-interactions. Male KHDRBS1 knockout mice are infertile due to aberrant differentiation of round spermatids into mature spermatozoa. A subset of testicular transcripts that are affected by KHDRBS1 ablation and found an enrichment in mRNAs encoding proteins involved in cell proliferation and survival has been identified. Several of these mRNAs are bound by KHDRBS1 in germ cells. Moreover, studies have provided evidence that, upon meiotic divisions, KHDRBS1 associates with the translation initiation complex and regulates polysomal loading and translation of the mRNAs encoding SPAG16, a cytoskeletal protein required for sperm motility and fertility; NEDD1, a centrosomal protein required for microtubule organization; and SPDYA, a cell cycle regulator. Findings suggest that KHDRBS1 loss of function leads to male infertility by restricting translation of a selected group of mRNA transcripts.
Publications
Asbach B, Ludwig C, Saksela K, Wagner R. Comprehensive analysis of interactions between the Src-associated protein in mitosis of 68 kDa and the human Src-homology 3 proteome. PLoS One. 2012;7(6):e38540. doi: 10.1371/journal.pone.0038540. Epub 2012 Jun 20. PMID: 22745667; PMCID: PMC3379994.
Enrica Bianchi, Federica Barbagallo, Claudia Valeri, Raffaele Geremia, Antonietta Salustri, Massimo De Felici, Claudio Sette, Ablation of the Sam68 gene impairs female fertility and gonadotropin-dependent follicle development, Human Molecular Genetics, Volume 19, Issue 24, 15 December 2010, Pages 4886–4894, https://doi.org/10.1093/hmg/ddq422
Paronetto MP, Messina V, Bianchi E, Barchi M, Vogel G, Moretti C, Palombi F, Stefanini M, Geremia R, Richard S, Sette C. Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J Cell Biol. 2009 Apr 20;185(2):235-49. doi: 10.1083/jcb.200811138. PMID: 19380878; PMCID: PMC2700383.
Lupeol
Summary
Lupeol is a small molecule that blocks the activation of CatSper, a key component of sperm function.
The Science
It is possible that lupeol occupies the steroid binding site of ABHD2, thus preventing CatSper activation by P4 via a competitive antagonist-type mechanism. Lupeol was also able to inhibit sperm hyperactivation and slightly reduced basal motility of capacitated sperm cells, as evident from computer-assisted sperm analyses (CASAs). Lupeol had no effect on sperm motility of non-capacitated cells, which indicates their low toxicity effect toward spermatozoa. (Learn more here.)
Publications
Brenker, C., Schiffer, C., Wagner, I. V., Tüttelmann, F., Röpke, A., Rennhack, A., . . . Strünker, T. (2018). Action of steroids and plant triterpenoids on CatSper Ca2 channels in human sperm. Proceedings of the National Academy of Sciences,115(3).
Mannowetz, N., Miller, M. R., & Lishko, P. V. (2017). Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proceedings of the National Academy of Sciences,114(22), 5743-5748.
Mannowetz, N., Mundt, N., & Lishko, P. V. (2018). Reply to Brenker et al.: The plant triterpenoid pristimerin inhibits calcium influx into human spermatozoa via CatSper. Proceedings of the National Academy of Sciences,115(3).
Lupeol is a small molecule that blocks the activation of CatSper, a key component of sperm function.
The Science
It is possible that lupeol occupies the steroid binding site of ABHD2, thus preventing CatSper activation by P4 via a competitive antagonist-type mechanism. Lupeol was also able to inhibit sperm hyperactivation and slightly reduced basal motility of capacitated sperm cells, as evident from computer-assisted sperm analyses (CASAs). Lupeol had no effect on sperm motility of non-capacitated cells, which indicates their low toxicity effect toward spermatozoa. (Learn more here.)
Publications
Brenker, C., Schiffer, C., Wagner, I. V., Tüttelmann, F., Röpke, A., Rennhack, A., . . . Strünker, T. (2018). Action of steroids and plant triterpenoids on CatSper Ca2 channels in human sperm. Proceedings of the National Academy of Sciences,115(3).
Mannowetz, N., Miller, M. R., & Lishko, P. V. (2017). Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proceedings of the National Academy of Sciences,114(22), 5743-5748.
Mannowetz, N., Mundt, N., & Lishko, P. V. (2018). Reply to Brenker et al.: The plant triterpenoid pristimerin inhibits calcium influx into human spermatozoa via CatSper. Proceedings of the National Academy of Sciences,115(3).
LY6K (Lymphocyte Antigen 6 Family Member K)
Summary
This protein is assumed to play an important role in facilitating a sperm’s navigation through the oviduct and its ability to fertilize an egg.
The Science
LY6K is a glycosylphosphatidylinositol (GPI)-anchored protein that is expressed specifically in the sperm. It is not expressed in mature spermatozoa; therefore, it could not be directly responsible for regulating sperm passage through the utero tubal junction and/or binding to the zona pellucida of cumulus-free eggs. Differing from more than 10 previously known gene knockout mouse lines that showed male infertility by impaired sperm migration into the oviduct, spermatozoa from Ly6K-/- mice had no aberrance in ADAM3. Thus, LY6K is a newly identified factor involved in sperm fertilizing ability. The lack of effect on ADAM3 in Ly6K-/- mice is indicative of an as yet undefined pathway in the mouse. The role of LY6K is considered to be independent from the endoplasmic reticulum chaperone quality control pathway for ADAM3. Interestingly, both TEX101 and Ly6k contribute mutually for their protein expression in the testicular germ cells, suggesting that the expression-balance of these two ADAM3-related testicular GPI- anchored proteins is essential for fertile sperm formation.
Publications
Endo, S., Yoshitake, H., Tsukamoto, H. et al. TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells. Sci Rep 6, 23616 (2016). https://doi.org/10.1038/srep23616
Fujihara Y, Okabe M, Ikawa M. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biology of Reproduction. 2014 Mar;90(3):60. DOI: 10.1095/biolreprod.113.112888. PMID: 24501175.
This protein is assumed to play an important role in facilitating a sperm’s navigation through the oviduct and its ability to fertilize an egg.
The Science
LY6K is a glycosylphosphatidylinositol (GPI)-anchored protein that is expressed specifically in the sperm. It is not expressed in mature spermatozoa; therefore, it could not be directly responsible for regulating sperm passage through the utero tubal junction and/or binding to the zona pellucida of cumulus-free eggs. Differing from more than 10 previously known gene knockout mouse lines that showed male infertility by impaired sperm migration into the oviduct, spermatozoa from Ly6K-/- mice had no aberrance in ADAM3. Thus, LY6K is a newly identified factor involved in sperm fertilizing ability. The lack of effect on ADAM3 in Ly6K-/- mice is indicative of an as yet undefined pathway in the mouse. The role of LY6K is considered to be independent from the endoplasmic reticulum chaperone quality control pathway for ADAM3. Interestingly, both TEX101 and Ly6k contribute mutually for their protein expression in the testicular germ cells, suggesting that the expression-balance of these two ADAM3-related testicular GPI- anchored proteins is essential for fertile sperm formation.
Publications
Endo, S., Yoshitake, H., Tsukamoto, H. et al. TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells. Sci Rep 6, 23616 (2016). https://doi.org/10.1038/srep23616
Fujihara Y, Okabe M, Ikawa M. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biology of Reproduction. 2014 Mar;90(3):60. DOI: 10.1095/biolreprod.113.112888. PMID: 24501175.
MAPK3/1 (Mitogen-activated Protein Kinase 3 and 1)
Summary
This protein has been shown to be a key component of meiosis during the development of new sperm cells (spermatogenesis).
The Science
MAP kinases act in a signaling cascade that regulates various cellular processes such as proliferation, differentiation, and cell cycle progression in response to a variety of extracellular signals. This kinase is activated by upstream kinases, resulting in its translocation to the nucleus where it phosphorylates nuclear targets. During mouse spermatogenesis, MAPK was shown to regulate the progression of prophase I to metaphase I during meiosis in germ cells. In the testes, almost 75% of the developing germ cells must undergo spontaneous apoptosis during normal spermatogenesis in mammals to maintain a constant ratio of the number of germ cells to Sertoli cells. MAPK has been implicated to play a role in germ cell apoptosis. Using isolated spermatozoa, a recent study reported the involvement of ERK1/2 and p38 MAPK in the regulation of forward and hyperactivated motility of sperm. In this study, it was reported that ERK becomes phosphorylated to promote motility, whereas p38 MAPK is phosphorylated to inhibit sperm motility. The same report also implicated the positive role of ERK and p38 MAPK in acrosome reaction.
Publications
Li MW, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009 Apr;15(4):159-68. doi: 10.1016/j.molmed.2009.02.002. Epub 2009 Mar 18. PMID: 19303360; PMCID: PMC2804913.
Troadec JD, Marien M, Mourlevat S, Debeir T, Ruberg M, Colpaert F, Michel PP. Activation of the mitogen-activated protein kinase (ERK(1/2)) signaling pathway by cyclic AMP potentiates the neuroprotective effect of the neurotransmitter noradrenaline on dopaminergic neurons. Mol Pharmacol. 2002 Nov;62(5):1043-52. doi: 10.1124/mol.62.5.1043. PMID: 12391266.
Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012 Aug;66(2):105-43. doi: 10.1016/j.phrs.2012.04.005. Epub 2012 Apr 27. PMID: 22569528.
This protein has been shown to be a key component of meiosis during the development of new sperm cells (spermatogenesis).
The Science
MAP kinases act in a signaling cascade that regulates various cellular processes such as proliferation, differentiation, and cell cycle progression in response to a variety of extracellular signals. This kinase is activated by upstream kinases, resulting in its translocation to the nucleus where it phosphorylates nuclear targets. During mouse spermatogenesis, MAPK was shown to regulate the progression of prophase I to metaphase I during meiosis in germ cells. In the testes, almost 75% of the developing germ cells must undergo spontaneous apoptosis during normal spermatogenesis in mammals to maintain a constant ratio of the number of germ cells to Sertoli cells. MAPK has been implicated to play a role in germ cell apoptosis. Using isolated spermatozoa, a recent study reported the involvement of ERK1/2 and p38 MAPK in the regulation of forward and hyperactivated motility of sperm. In this study, it was reported that ERK becomes phosphorylated to promote motility, whereas p38 MAPK is phosphorylated to inhibit sperm motility. The same report also implicated the positive role of ERK and p38 MAPK in acrosome reaction.
Publications
Li MW, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009 Apr;15(4):159-68. doi: 10.1016/j.molmed.2009.02.002. Epub 2009 Mar 18. PMID: 19303360; PMCID: PMC2804913.
Troadec JD, Marien M, Mourlevat S, Debeir T, Ruberg M, Colpaert F, Michel PP. Activation of the mitogen-activated protein kinase (ERK(1/2)) signaling pathway by cyclic AMP potentiates the neuroprotective effect of the neurotransmitter noradrenaline on dopaminergic neurons. Mol Pharmacol. 2002 Nov;62(5):1043-52. doi: 10.1124/mol.62.5.1043. PMID: 12391266.
Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012 Aug;66(2):105-43. doi: 10.1016/j.phrs.2012.04.005. Epub 2012 Apr 27. PMID: 22569528.
The MAPK Signaling Pathway
PAEP (Progestogen-associated Endometrial Protein)
Summary
This protein appears to play a key role in facilitating the fusion of a sperm to an egg, thereby making it critical in the fertilization process.
The Science
PAEP (previously known as Glycodelin) is an example of a glycoprotein whose complex-type glycans mediate biological actions in human reproduction and immune reactions. Being attached to an identical protein backbone, PAEP oligosaccharides vary significantly from one reproductive tissue to another and have an effect on its own secretion and role in cell communication. For instance, uterine PAEP-A inhibits sperm–oocyte interaction by binding on the sperm head. This is a glycosylation-dependent phenomenon, in which fucosyltransferase-5 plays a key role. PAEP-S from seminal plasma binds evenly around the sperm head and maintains an uncapacitated state in the spermatozoa, until the isoform is detached during sperm passage through the cervix. PAEP-F from follicular fluid and Fallopian tube binds to the acrosomal region of the sperm head, thereby inhibiting both the sperm–oocyte binding and premature progesterone-induced acrosome reaction. The cumulus cells surrounding the oocyte can capture PAEP-A and -F from the surrounding environment and convert these isoforms to a cumulus cell isoform, PAEP-C. It differs by glycosylation from the other isoforms, and it too attaches on the sperm head, with the highest density in the equatorial region. PAEP-C is capable of detaching the sperm-bound inhibitory isoforms so that the sperm–oocyte binding is enhanced. PAEP-A also has immunosuppressive actions directed to cellular, humoral and innate immunity. Although these actions depend mainly on the protein backbone, glycosylation also plays a part. Glycosylated PAEP may be involved in the protection of spermatozoa against maternal immune reactions, and PAEP also has apoptogenic activity. Some glycosylation patterns of PAEP may mask its apoptogenic domain.
Publications
M. Seppälä, H. Koistinen, R. Koistinen, P.C.N. Chiu, W.S.B. Yeung, Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations, Human Reproduction Update, Volume 13, Issue 3, May/June 2007, Pages 275–287, https://doi.org/10.1093/humupd/dmm004
Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002 Aug;23(4):401-30. doi: 10.1210/er.2001-0026. PMID: 12202458.
Yeung WS, Lee KF, Koistinen R, Koistinen H, Seppälä M, Chiu PC. Effects of glycodelins on functional competence of spermatozoa. J Reprod Immunol. 2009 Dec;83(1-2):26-30. doi: 10.1016/j.jri.2009.04.012. Epub 2009 Oct 25. PMID: 19857900.
This protein appears to play a key role in facilitating the fusion of a sperm to an egg, thereby making it critical in the fertilization process.
The Science
PAEP (previously known as Glycodelin) is an example of a glycoprotein whose complex-type glycans mediate biological actions in human reproduction and immune reactions. Being attached to an identical protein backbone, PAEP oligosaccharides vary significantly from one reproductive tissue to another and have an effect on its own secretion and role in cell communication. For instance, uterine PAEP-A inhibits sperm–oocyte interaction by binding on the sperm head. This is a glycosylation-dependent phenomenon, in which fucosyltransferase-5 plays a key role. PAEP-S from seminal plasma binds evenly around the sperm head and maintains an uncapacitated state in the spermatozoa, until the isoform is detached during sperm passage through the cervix. PAEP-F from follicular fluid and Fallopian tube binds to the acrosomal region of the sperm head, thereby inhibiting both the sperm–oocyte binding and premature progesterone-induced acrosome reaction. The cumulus cells surrounding the oocyte can capture PAEP-A and -F from the surrounding environment and convert these isoforms to a cumulus cell isoform, PAEP-C. It differs by glycosylation from the other isoforms, and it too attaches on the sperm head, with the highest density in the equatorial region. PAEP-C is capable of detaching the sperm-bound inhibitory isoforms so that the sperm–oocyte binding is enhanced. PAEP-A also has immunosuppressive actions directed to cellular, humoral and innate immunity. Although these actions depend mainly on the protein backbone, glycosylation also plays a part. Glycosylated PAEP may be involved in the protection of spermatozoa against maternal immune reactions, and PAEP also has apoptogenic activity. Some glycosylation patterns of PAEP may mask its apoptogenic domain.
Publications
M. Seppälä, H. Koistinen, R. Koistinen, P.C.N. Chiu, W.S.B. Yeung, Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations, Human Reproduction Update, Volume 13, Issue 3, May/June 2007, Pages 275–287, https://doi.org/10.1093/humupd/dmm004
Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002 Aug;23(4):401-30. doi: 10.1210/er.2001-0026. PMID: 12202458.
Yeung WS, Lee KF, Koistinen R, Koistinen H, Seppälä M, Chiu PC. Effects of glycodelins on functional competence of spermatozoa. J Reprod Immunol. 2009 Dec;83(1-2):26-30. doi: 10.1016/j.jri.2009.04.012. Epub 2009 Oct 25. PMID: 19857900.
PDILT (Protein Disulfide Isomerase Like, Testis Expressed)
Summary
This protein is believed to be a necessary component of sperm maturation, meaning impeding their function could induce infertility.
The Science
PDILT is a member of the disulfide isomerase family of endoplasmic reticulum proteins that catalyze protein folding and thiol-disulfide interchange reactions. Appropriate intra- and intermolecular disulfide bond formation also promotes protein folding in the ER. For disulfide bond formation in the ER, more than 20 protein disulfide isomerase (PDI) family proteins have been implicated in the process of oxidation, reduction, and isomerization. In addition to ubiquitous PDIs including PDIA3, male germ cells also express a testis specific PDI-like protein, PDILT. Although PDILT does not have consensus CXXC motifs and is not a classical oxidoreductase, it does interact with CLGN and with model proteins in vitro, suggesting that it may have a role in the maturation of spermatid proteins and consequently, male infertility in vivo. A study showed that appropriate disulfide formation in ADAM3 relies on a CALR3/PDILT partnership, and that PDILT was not detectable until the mice became 3 weeks old, indicating postmeiotic expression. Although PDILT lacks a redox-active CXXC motif, the fact that PDILT can be recovered in disulfide-dependent complexes suggests that the monocysteinic thioredoxin motif may trap cysteine in the substrate to facilitate protein folding. PDILT is not active as a reductase against the model substrate insulin.
Publications
Li H, Yang K, Wang W, et al. Crystal and solution structures of human protein-disulfide isomerase-like protein of the testis (PDILT) provide insight into its chaperone activity. J Biol Chem. 2018;293(4):1192-1202. doi:10.1074/jbc.M117.797290
Tokuhiro K, Ikawa M, Benham AM, Okabe M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]. Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3850-5. doi: 10.1073/pnas.1117963109. Epub 2012 Feb 22. Erratum in: Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5905. PMID: 22357757; PMCID: PMC3309714.
Yamaguchi R, Fujihara Y, Ikawa M, Okabe M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol Biol Cell. 2012 Jul;23(14):2671-9. doi: 10.1091/mbc.E11-12-1025. Epub 2012 May 23. PMID: 22621904; PMCID: PMC3395656.
This protein is believed to be a necessary component of sperm maturation, meaning impeding their function could induce infertility.
The Science
PDILT is a member of the disulfide isomerase family of endoplasmic reticulum proteins that catalyze protein folding and thiol-disulfide interchange reactions. Appropriate intra- and intermolecular disulfide bond formation also promotes protein folding in the ER. For disulfide bond formation in the ER, more than 20 protein disulfide isomerase (PDI) family proteins have been implicated in the process of oxidation, reduction, and isomerization. In addition to ubiquitous PDIs including PDIA3, male germ cells also express a testis specific PDI-like protein, PDILT. Although PDILT does not have consensus CXXC motifs and is not a classical oxidoreductase, it does interact with CLGN and with model proteins in vitro, suggesting that it may have a role in the maturation of spermatid proteins and consequently, male infertility in vivo. A study showed that appropriate disulfide formation in ADAM3 relies on a CALR3/PDILT partnership, and that PDILT was not detectable until the mice became 3 weeks old, indicating postmeiotic expression. Although PDILT lacks a redox-active CXXC motif, the fact that PDILT can be recovered in disulfide-dependent complexes suggests that the monocysteinic thioredoxin motif may trap cysteine in the substrate to facilitate protein folding. PDILT is not active as a reductase against the model substrate insulin.
Publications
Li H, Yang K, Wang W, et al. Crystal and solution structures of human protein-disulfide isomerase-like protein of the testis (PDILT) provide insight into its chaperone activity. J Biol Chem. 2018;293(4):1192-1202. doi:10.1074/jbc.M117.797290
Tokuhiro K, Ikawa M, Benham AM, Okabe M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]. Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3850-5. doi: 10.1073/pnas.1117963109. Epub 2012 Feb 22. Erratum in: Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5905. PMID: 22357757; PMCID: PMC3309714.
Yamaguchi R, Fujihara Y, Ikawa M, Okabe M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol Biol Cell. 2012 Jul;23(14):2671-9. doi: 10.1091/mbc.E11-12-1025. Epub 2012 May 23. PMID: 22621904; PMCID: PMC3395656.
Protein Phosphatases
Summary
This protein family is believed to play a key role in facilitating sperm motility, as well as capacitation (the change sperm undergo in the female reproductive tract that enables them to penetrate and fertilize an egg.)
The Science
Protein phosphatase 1 (PPI) is a major serine/threonine-PP, for which >200 interactors (PP1 interacting proteins—PIPs) have been identified that control its activity, subcellular location and substrate specificity. For PP1, several isoforms have been described, among them PP1γ2, a testis/sperm-enriched PP1 isoform. Recent findings support the hypothesis that PP1γ2 is involved in the regulation of sperm motility. Inhibition of PPs induces capacitation-associated signalling, and immotile spermatozoa possess higher activity levels of PP1γ2 compared with motile spermatozoa. Inhibition of PP activity by okadaic acid and calyculin A initiates motility in caput epididymal sperm without requirement for a change in cyclic AMP (cAMP) levels. Globally, 128 PP1-PIPs have been described in the literature. Of these, four are testis and sperm-specific PP1-PIP complexes and have conclusively been well documented in the literature.
Publications
Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015 Jul-Aug;21(4):455-85. doi: 10.1093/humupd/dmv020. Epub 2015 Apr 17. PMID: 25888788.
Margarida Fardilha, Sara L.C. Esteves, Luís Korrodi-Gregório, Steven Pelech, Odete A.B. da Cruz e Silva, Edgar da Cruz e Silva, Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility, Molecular Human Reproduction, Volume 17, Issue 8, August 2011, Pages 466–477, https://doi.org/10.1093/molehr/gar004
Fardilha M, Esteves SL, Korrodi-Gregório L, Vintém AP, Domingues SC, Rebelo S, Morrice N, Cohen PT, da Cruz e Silva OA, da Cruz e Silva EF. Identification of the human testis protein phosphatase 1 interactome. Biochem Pharmacol. 2011 Nov 15;82(10):1403-15. doi: 10.1016/j.bcp.2011.02.018. Epub 2011 Mar 5. PMID: 21382349.
This protein family is believed to play a key role in facilitating sperm motility, as well as capacitation (the change sperm undergo in the female reproductive tract that enables them to penetrate and fertilize an egg.)
The Science
Protein phosphatase 1 (PPI) is a major serine/threonine-PP, for which >200 interactors (PP1 interacting proteins—PIPs) have been identified that control its activity, subcellular location and substrate specificity. For PP1, several isoforms have been described, among them PP1γ2, a testis/sperm-enriched PP1 isoform. Recent findings support the hypothesis that PP1γ2 is involved in the regulation of sperm motility. Inhibition of PPs induces capacitation-associated signalling, and immotile spermatozoa possess higher activity levels of PP1γ2 compared with motile spermatozoa. Inhibition of PP activity by okadaic acid and calyculin A initiates motility in caput epididymal sperm without requirement for a change in cyclic AMP (cAMP) levels. Globally, 128 PP1-PIPs have been described in the literature. Of these, four are testis and sperm-specific PP1-PIP complexes and have conclusively been well documented in the literature.
Publications
Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015 Jul-Aug;21(4):455-85. doi: 10.1093/humupd/dmv020. Epub 2015 Apr 17. PMID: 25888788.
Margarida Fardilha, Sara L.C. Esteves, Luís Korrodi-Gregório, Steven Pelech, Odete A.B. da Cruz e Silva, Edgar da Cruz e Silva, Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility, Molecular Human Reproduction, Volume 17, Issue 8, August 2011, Pages 466–477, https://doi.org/10.1093/molehr/gar004
Fardilha M, Esteves SL, Korrodi-Gregório L, Vintém AP, Domingues SC, Rebelo S, Morrice N, Cohen PT, da Cruz e Silva OA, da Cruz e Silva EF. Identification of the human testis protein phosphatase 1 interactome. Biochem Pharmacol. 2011 Nov 15;82(10):1403-15. doi: 10.1016/j.bcp.2011.02.018. Epub 2011 Mar 5. PMID: 21382349.
SEMG1 (Semenogelin)
Summary
This protein is believed to play both a role in facilitating sperm motility as well as protecting sperm within the acidic environment of the female reproductive tract.
The Science
(Note: SEMG1 and SEMG2 are paralogs with similar activity and expression patterns and are collectively referred to as SEMG in the following description.) SEMG1 and 2 are the predominant structural proteins in the loose gel formed in freshly ejaculated human semen. The concentration of SEMG1 is five- to ten-fold higher than the level of SEMG2 in semen, and these two molecules are the quantitatively dominating proteins in the fluid from the seminal vesicles. The semen coagulum liquefies spontaneously within minutes after ejaculation, and to date, the prostate-specific antigen (PSA), a protease, appears to be the enzyme that specifically processes SEMG. The main and temporally first role of SEMG in the reproductive process is related to the formation of the seminal coagulum. SEMG is also a potent activator of sperm hyaluronidase, suggesting a potential participation of SEMG in the degradation of the egg’s envelope at fertilization and sperm penetration. SEMG and its PSA digest have also been found to contribute significantly to the high antibacterial activity of seminal plasma. In relation with this function, SEMG associates with Eppin that is adsorbed at the surface of spermatozoa. Eppin is a cysteine-rich, androgen- regulated protein that originates only from the testis and epididymis and has antimicrobial activity. The resulting complex, as well as Eppin and SEMG-derived peptides, appear to provide an antimicrobial activity that may help the survival of spermatozoa in the female reproductive tract. In addition, EPPIN controls sperm motility in the ejaculate by binding SEMG1, resulting in the loss of calcium, most likely through a disturbance of internal pH and an inhibition of uptake mechanisms. However, the exact steps through which the EPPIN-SEMG1 complex exerts its effect on internal calcium levels are unknown. Anti-EPPIN antibodies can substitute for SEMG1, and, therefore, small-molecular weight compounds that mimic anti-EPPIN binding should be able to substitute for SEMG1, providing the basis for a nonantibody, non-hormonal male contraceptive.
Publications
de Lamirande E. Semenogelin, the main protein of the human semen coagulum, regulates sperm function. Semin Thromb Hemost. 2007 Feb;33(1):60-8. doi: 10.1055/s-2006-958463. PMID: 17253191.
Lilja H, Lundwall A. Molecular cloning of epididymal and seminal vesicular transcripts encoding a semenogelin-related protein. Proc Natl Acad Sci U S A. 1992;89(10):4559-4563. doi:10.1073/pnas.89.10.4559
Malm J, Jonsson M, Frohm B, Linse S. Structural properties of semenogelin I. FEBS J. 2007 Sep;274(17):4503-10. doi: 10.1111/j.1742-4658.2007.05979.x. Epub 2007 Aug 3. PMID: 17680810.
This protein is believed to play both a role in facilitating sperm motility as well as protecting sperm within the acidic environment of the female reproductive tract.
The Science
(Note: SEMG1 and SEMG2 are paralogs with similar activity and expression patterns and are collectively referred to as SEMG in the following description.) SEMG1 and 2 are the predominant structural proteins in the loose gel formed in freshly ejaculated human semen. The concentration of SEMG1 is five- to ten-fold higher than the level of SEMG2 in semen, and these two molecules are the quantitatively dominating proteins in the fluid from the seminal vesicles. The semen coagulum liquefies spontaneously within minutes after ejaculation, and to date, the prostate-specific antigen (PSA), a protease, appears to be the enzyme that specifically processes SEMG. The main and temporally first role of SEMG in the reproductive process is related to the formation of the seminal coagulum. SEMG is also a potent activator of sperm hyaluronidase, suggesting a potential participation of SEMG in the degradation of the egg’s envelope at fertilization and sperm penetration. SEMG and its PSA digest have also been found to contribute significantly to the high antibacterial activity of seminal plasma. In relation with this function, SEMG associates with Eppin that is adsorbed at the surface of spermatozoa. Eppin is a cysteine-rich, androgen- regulated protein that originates only from the testis and epididymis and has antimicrobial activity. The resulting complex, as well as Eppin and SEMG-derived peptides, appear to provide an antimicrobial activity that may help the survival of spermatozoa in the female reproductive tract. In addition, EPPIN controls sperm motility in the ejaculate by binding SEMG1, resulting in the loss of calcium, most likely through a disturbance of internal pH and an inhibition of uptake mechanisms. However, the exact steps through which the EPPIN-SEMG1 complex exerts its effect on internal calcium levels are unknown. Anti-EPPIN antibodies can substitute for SEMG1, and, therefore, small-molecular weight compounds that mimic anti-EPPIN binding should be able to substitute for SEMG1, providing the basis for a nonantibody, non-hormonal male contraceptive.
Publications
de Lamirande E. Semenogelin, the main protein of the human semen coagulum, regulates sperm function. Semin Thromb Hemost. 2007 Feb;33(1):60-8. doi: 10.1055/s-2006-958463. PMID: 17253191.
Lilja H, Lundwall A. Molecular cloning of epididymal and seminal vesicular transcripts encoding a semenogelin-related protein. Proc Natl Acad Sci U S A. 1992;89(10):4559-4563. doi:10.1073/pnas.89.10.4559
Malm J, Jonsson M, Frohm B, Linse S. Structural properties of semenogelin I. FEBS J. 2007 Sep;274(17):4503-10. doi: 10.1111/j.1742-4658.2007.05979.x. Epub 2007 Aug 3. PMID: 17680810.
SLC9C1 (Solute Carrier Family 9, member C1)
Summary
This family of proteins appears to be integral for sperm motility.
The Science
SLCs are a family of integral membrane proteins that mediate electroneutral exchange of Na+ for H+ across the plasma membrane and have important roles in intracellular pH (pHi) regulation. Among numerous SLCs, SLC9C1 and NHA1 are specifically distributed in the principal piece of the sperm flagellum. Studies have shown that the SLC9C1-null spermatozoa fail to develop the cAMP- dependent protein tyrosine phosphorylation that coincides with the functional maturation occurring upon incubation in capacitating conditions in vitro. Both the sperm motility defect and the lack of induced protein tyrosine phosphorylation are rescued by the addition of cell-permeable cAMP analogs, suggesting that cAMP metabolism is impaired in spermatozoa lacking SLC9C1. Evidence has shown that a molecular complex including both SLC9C1 and sAC is assembled in mouse spermatozoa. SLC9C1 appears to modulate cellular sAC activity on multiple levels, including the stable expression of sACfl, the regulation of bicarbonate transport possibly through an associated transporter, and by modulating local pH for optimal sAC activity. In a reciprocal manner, SLC9C1 activity and/or ion-exchange function(s) may be modulated by sAC activity through the putative cyclic nucleotide binding motif in the SLC9C1 cytoplasmic carboxyl tail. Finally, carbonic anhydrase II binds to the cytoplasmic carboxyl tail of NHE1. It has been proposed that a similar association of carbonic anhydrase II with the SLC9C1 cytoplasmic tail may exist. Although speculative, such a signaling complex could very efficiently regulate intracellular pH, bicarbonate, and cAMP levels, enabling the rapid and precise control of both sAC and SLC9C1 activities to facilitate sperm motility regulation. The last four predicted transmembrane segments of the new SLC are also highly similar to apparent transmembrane segments found in the CatSpers family of sperm voltage-gated cation channels.
Publications
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016 Nov 10;7(11):e2472. doi: 10.1038/cddis.2016.344. PMID: 27831554; PMCID: PMC5260884.
Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003 Dec;5(12):1117-22. doi: 10.1038/ncb1072. Epub 2003 Nov 23. PMID: 14634667.
Windler, F., Bönigk, W., Körschen, H.G. et al. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat Commun 9, 2809 (2018). https://doi.org/10.1038/s41467-018-05253-x
This family of proteins appears to be integral for sperm motility.
The Science
SLCs are a family of integral membrane proteins that mediate electroneutral exchange of Na+ for H+ across the plasma membrane and have important roles in intracellular pH (pHi) regulation. Among numerous SLCs, SLC9C1 and NHA1 are specifically distributed in the principal piece of the sperm flagellum. Studies have shown that the SLC9C1-null spermatozoa fail to develop the cAMP- dependent protein tyrosine phosphorylation that coincides with the functional maturation occurring upon incubation in capacitating conditions in vitro. Both the sperm motility defect and the lack of induced protein tyrosine phosphorylation are rescued by the addition of cell-permeable cAMP analogs, suggesting that cAMP metabolism is impaired in spermatozoa lacking SLC9C1. Evidence has shown that a molecular complex including both SLC9C1 and sAC is assembled in mouse spermatozoa. SLC9C1 appears to modulate cellular sAC activity on multiple levels, including the stable expression of sACfl, the regulation of bicarbonate transport possibly through an associated transporter, and by modulating local pH for optimal sAC activity. In a reciprocal manner, SLC9C1 activity and/or ion-exchange function(s) may be modulated by sAC activity through the putative cyclic nucleotide binding motif in the SLC9C1 cytoplasmic carboxyl tail. Finally, carbonic anhydrase II binds to the cytoplasmic carboxyl tail of NHE1. It has been proposed that a similar association of carbonic anhydrase II with the SLC9C1 cytoplasmic tail may exist. Although speculative, such a signaling complex could very efficiently regulate intracellular pH, bicarbonate, and cAMP levels, enabling the rapid and precise control of both sAC and SLC9C1 activities to facilitate sperm motility regulation. The last four predicted transmembrane segments of the new SLC are also highly similar to apparent transmembrane segments found in the CatSpers family of sperm voltage-gated cation channels.
Publications
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail. Cell Death Dis. 2016 Nov 10;7(11):e2472. doi: 10.1038/cddis.2016.344. PMID: 27831554; PMCID: PMC5260884.
Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003 Dec;5(12):1117-22. doi: 10.1038/ncb1072. Epub 2003 Nov 23. PMID: 14634667.
Windler, F., Bönigk, W., Körschen, H.G. et al. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat Commun 9, 2809 (2018). https://doi.org/10.1038/s41467-018-05253-x
SLC26A8 (Solute Carrier Family 26 Member 8)
Summary
This protein coding gene is believed to play a critical role in the development of sperm cells, specifically the flagella (or tail): mammals lacking this have displayed severely deformed sperm tails, which are incapable of normal functioning.
The Science
In SLC26A8 null mice, epididymal sperm display striking morphological abnormalities consisting of a thinning of the flagella at the end of the midpiece and abnormal angulation of the sperm tail, which was folded at 90 or 180 degrees. Flagella thinning was found in the testis, but angulation appeared in epididymis and increased from the caput to the cauda. Ultra-structurally, spermatogenesis appeared normal until the early stage of elongating spermatids; the first anomalies were observed at annulus relocation, which failed to reach the midpiece-principal piece junction, leading to an incomplete mitochondrial sheath. Accordingly, a ‘gap’ between the midpiece and the principal piece has been observed that most likely corresponds to the terminal segment of the midpiece without the surrounding mitochondria. In the epididymis, flagella were radically bent and displayed a hairpin configuration with the annulus either abnormally shaped, ectopically located, or isolated from other flagellar structures. The retroflexion occurred in the gap region, leading to a disruption of the axial structures (the axoneme and dense fibres). The annulus had an oval shape and was linked only to the mitochondria as opposed to the normal triangular shaped structure linked to the mitochondria, the fibrous sheath, and the plasma membrane. Finally, the midpiece appeared disorganised, with unequally sized mitochondria that had failed to establish the usual regular helical pattern. Despite these flagella abnormalities, protein expression levels of flagella-constitutive proteins (dynein intermediate chain, actin, septins 1, 4, 6 and 7, cytochrome C) in SLC26A8 null epididymes were normal.
Publications
Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Aspects Med. 2013;34(2-3):494-515. doi:10.1016/j.mam.2012.07.009
Dirami T, Rode B, Jollivet M, Da Silva N, Escalier D, Gaitch N, Norez C, Tuffery P, Wolf JP, Becq F, Ray PF, Dulioust E, Gacon G, Bienvenu T, Touré A. Missense mutations in SLC26A8, encoding a sperm-specific activator of CFTR, are associated with human asthenozoospermia. Am J Hum Genet. 2013 May 2;92(5):760-6. doi: 10.1016/j.ajhg.2013.03.016. Epub 2013 Apr 11. PMID: 23582645; PMCID: PMC3644633.
Li J, Xia F, Reithmeier RA. N-glycosylation and topology of the human SLC26 family of anion transport membrane proteins. Am J Physiol Cell Physiol. 2014 May 15;306(10):C943-60. doi: 10.1152/ajpcell.00030.2014. Epub 2014 Mar 19. PMID: 24647542.
This protein coding gene is believed to play a critical role in the development of sperm cells, specifically the flagella (or tail): mammals lacking this have displayed severely deformed sperm tails, which are incapable of normal functioning.
The Science
In SLC26A8 null mice, epididymal sperm display striking morphological abnormalities consisting of a thinning of the flagella at the end of the midpiece and abnormal angulation of the sperm tail, which was folded at 90 or 180 degrees. Flagella thinning was found in the testis, but angulation appeared in epididymis and increased from the caput to the cauda. Ultra-structurally, spermatogenesis appeared normal until the early stage of elongating spermatids; the first anomalies were observed at annulus relocation, which failed to reach the midpiece-principal piece junction, leading to an incomplete mitochondrial sheath. Accordingly, a ‘gap’ between the midpiece and the principal piece has been observed that most likely corresponds to the terminal segment of the midpiece without the surrounding mitochondria. In the epididymis, flagella were radically bent and displayed a hairpin configuration with the annulus either abnormally shaped, ectopically located, or isolated from other flagellar structures. The retroflexion occurred in the gap region, leading to a disruption of the axial structures (the axoneme and dense fibres). The annulus had an oval shape and was linked only to the mitochondria as opposed to the normal triangular shaped structure linked to the mitochondria, the fibrous sheath, and the plasma membrane. Finally, the midpiece appeared disorganised, with unequally sized mitochondria that had failed to establish the usual regular helical pattern. Despite these flagella abnormalities, protein expression levels of flagella-constitutive proteins (dynein intermediate chain, actin, septins 1, 4, 6 and 7, cytochrome C) in SLC26A8 null epididymes were normal.
Publications
Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Aspects Med. 2013;34(2-3):494-515. doi:10.1016/j.mam.2012.07.009
Dirami T, Rode B, Jollivet M, Da Silva N, Escalier D, Gaitch N, Norez C, Tuffery P, Wolf JP, Becq F, Ray PF, Dulioust E, Gacon G, Bienvenu T, Touré A. Missense mutations in SLC26A8, encoding a sperm-specific activator of CFTR, are associated with human asthenozoospermia. Am J Hum Genet. 2013 May 2;92(5):760-6. doi: 10.1016/j.ajhg.2013.03.016. Epub 2013 Apr 11. PMID: 23582645; PMCID: PMC3644633.
Li J, Xia F, Reithmeier RA. N-glycosylation and topology of the human SLC26 family of anion transport membrane proteins. Am J Physiol Cell Physiol. 2014 May 15;306(10):C943-60. doi: 10.1152/ajpcell.00030.2014. Epub 2014 Mar 19. PMID: 24647542.
SPACA1 (Sperm Acrosome-associated 1)
Summary
Deficiencies in this protein have led to the development of sperm with misshapen heads, incapable of fertilizing an egg.
The Science
SPACA1 is a membrane protein localized in the mammalian spermatozoa that is reported to function in sperm-egg fusion. SPACA1 is recognized by anti-sperm antibodies from infertile males. Furthermore, antibodies generated against the recombinant protein block in vitro fertilization. This protein localizes to the acrosomal membrane of spermatids and mature spermatozoa where it is thought to play a role in acrosomal morphogenesis and in sperm-egg binding and fusion, respectively. SPACA1 is an integral acrosomal membrane protein, indicating that the major cause of globozoospermia is not aberrant protein transport in the Golgi or into the acrosome. Rather, the globozoospermia in Gopc- and Zpbp1-disrupted mouse lines is due to the loss of SPACA1 from their acrosomes. Disruption of Spaca1 led to poor formation of intermediate filament bundles, which normally form a characteristic dense structure on the nuclear membrane facing the inner acrosomal membrane. Impaired acrosome- acroplaxome-manchette formation might be involved in the cascades that lead to globular-headed spermatozoa. This impaired complex formation does not seem to cause severe abnormalities in mitochondrial shape and sperm tail formation during spermatogenesis, but abnormal coiling of the tail around the sperm head together with misshapen mitochondria became prominent during sperm passage thorough the epididymis. The same phenomenon was also reported in both Gopc- and Zpbp1-disrupted spermatozoa. The reasons for the progression of such abnormalities in the epididymis remain to be clarified.
Publications
Fujihara Y, Satouh Y, Inoue N, Isotani A, Ikawa M, Okabe M. SPACA1-deficient male mice are infertile with abnormally shaped sperm heads reminiscent of globozoospermia. Development. 2012 Oct;139(19):3583-9. doi: 10.1242/dev.081778. PMID: 22949614.
Kishida K, Harayama H, Kimura F, Murakami T. Individual differences in the distribution of sperm acrosome-associated 1 proteins among male patients of infertile couples; their possible impact on outcomes of conventional in vitro fertilization. Zygote. 2016 Oct;24(5):654-61. doi: 10.1017/S0967199415000623. Epub 2016 May 17. PMID: 27185107.
Yamatoya K, Kousaka M, Ito C, Nakata K, Hatano M, Araki Y, Toshimori K. Cleavage of SPACA1 regulates assembly of sperm-egg membrane fusion machinery in mature spermatozoa†. Biol Reprod. 2020 Mar 13;102(3):750-757. doi: 10.1093/biolre/ioz223. PMID: 31836887.
Deficiencies in this protein have led to the development of sperm with misshapen heads, incapable of fertilizing an egg.
The Science
SPACA1 is a membrane protein localized in the mammalian spermatozoa that is reported to function in sperm-egg fusion. SPACA1 is recognized by anti-sperm antibodies from infertile males. Furthermore, antibodies generated against the recombinant protein block in vitro fertilization. This protein localizes to the acrosomal membrane of spermatids and mature spermatozoa where it is thought to play a role in acrosomal morphogenesis and in sperm-egg binding and fusion, respectively. SPACA1 is an integral acrosomal membrane protein, indicating that the major cause of globozoospermia is not aberrant protein transport in the Golgi or into the acrosome. Rather, the globozoospermia in Gopc- and Zpbp1-disrupted mouse lines is due to the loss of SPACA1 from their acrosomes. Disruption of Spaca1 led to poor formation of intermediate filament bundles, which normally form a characteristic dense structure on the nuclear membrane facing the inner acrosomal membrane. Impaired acrosome- acroplaxome-manchette formation might be involved in the cascades that lead to globular-headed spermatozoa. This impaired complex formation does not seem to cause severe abnormalities in mitochondrial shape and sperm tail formation during spermatogenesis, but abnormal coiling of the tail around the sperm head together with misshapen mitochondria became prominent during sperm passage thorough the epididymis. The same phenomenon was also reported in both Gopc- and Zpbp1-disrupted spermatozoa. The reasons for the progression of such abnormalities in the epididymis remain to be clarified.
Publications
Fujihara Y, Satouh Y, Inoue N, Isotani A, Ikawa M, Okabe M. SPACA1-deficient male mice are infertile with abnormally shaped sperm heads reminiscent of globozoospermia. Development. 2012 Oct;139(19):3583-9. doi: 10.1242/dev.081778. PMID: 22949614.
Kishida K, Harayama H, Kimura F, Murakami T. Individual differences in the distribution of sperm acrosome-associated 1 proteins among male patients of infertile couples; their possible impact on outcomes of conventional in vitro fertilization. Zygote. 2016 Oct;24(5):654-61. doi: 10.1017/S0967199415000623. Epub 2016 May 17. PMID: 27185107.
Yamatoya K, Kousaka M, Ito C, Nakata K, Hatano M, Araki Y, Toshimori K. Cleavage of SPACA1 regulates assembly of sperm-egg membrane fusion machinery in mature spermatozoa†. Biol Reprod. 2020 Mar 13;102(3):750-757. doi: 10.1093/biolre/ioz223. PMID: 31836887.
SPAG16 (Sperm Associated Antigen 16)
Summary
This antigen encodes proteins responsible for the development of the central strand of the sperm’s tail.
The Science
Cilia and flagella are composed of a microtubular backbone, the axoneme, which is organized by the basal body and surrounded by plasma membrane. SPAG16 encodes 2 major proteins that associate with the axoneme of sperm tail and the nucleus of postmeiotic germ cells, respectively. In mice, the meiotically-expressed SPAG16L is found in the cytoplasm of germ cells and then becomes part of the central apparatus of the sperm axoneme. In contrast, the SPAG16S protein is expressed postmeiotically and accumulates in nuclei of spermatids. The absence of a severe spermatogenic defect in this SPAG16L mouse model indicates that it is SPAG16S that causes the serious deficiency in spermatogenesis seen in males in which both SPAG16L and SPAG16S were eliminated. The mild spermatogenetic defects in SPAG16L null mice suggest that SPAG16L is involved in nuclear remodeling that occur during spermatogenesis. The male infertility phenotype caused by a marked reduction in sperm motility and the absence of gross abnormalities in tissues containing ciliated cells indicates that SPAG16L could be a good molecular target for male contraception.
Publications
Nagarkatti-Gude DR, Jaimez R, Henderson SC, Teves ME, Zhang Z, Strauss JF 3rd. Spag16, an axonemal central apparatus gene, encodes a male germ cell nuclear speckle protein that regulates SPAG16 mRNA expression. PLoS One. 2011;6(5):e20625. doi: 10.1371/journal.pone.0020625. Epub 2011 May 31. PMID: 21655194; PMCID: PMC3105110.
Zhang Z, Kostetskii I, Moss SB, et al. Haploinsufficiency for the murine orthologue of Chlamydomonas PF20 disrupts spermatogenesis. Proc Natl Acad Sci U S A. 2004;101(35):12946-12951. doi:10.1073/pnas.0404280101
Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL, Moss SB, Radice GL, Strauss JF 3rd. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod. 2006 Apr;74(4):751-9. doi: 10.1095/biolreprod.105.049254. Epub 2005 Dec 28. PMID: 16382026.
This antigen encodes proteins responsible for the development of the central strand of the sperm’s tail.
The Science
Cilia and flagella are composed of a microtubular backbone, the axoneme, which is organized by the basal body and surrounded by plasma membrane. SPAG16 encodes 2 major proteins that associate with the axoneme of sperm tail and the nucleus of postmeiotic germ cells, respectively. In mice, the meiotically-expressed SPAG16L is found in the cytoplasm of germ cells and then becomes part of the central apparatus of the sperm axoneme. In contrast, the SPAG16S protein is expressed postmeiotically and accumulates in nuclei of spermatids. The absence of a severe spermatogenic defect in this SPAG16L mouse model indicates that it is SPAG16S that causes the serious deficiency in spermatogenesis seen in males in which both SPAG16L and SPAG16S were eliminated. The mild spermatogenetic defects in SPAG16L null mice suggest that SPAG16L is involved in nuclear remodeling that occur during spermatogenesis. The male infertility phenotype caused by a marked reduction in sperm motility and the absence of gross abnormalities in tissues containing ciliated cells indicates that SPAG16L could be a good molecular target for male contraception.
Publications
Nagarkatti-Gude DR, Jaimez R, Henderson SC, Teves ME, Zhang Z, Strauss JF 3rd. Spag16, an axonemal central apparatus gene, encodes a male germ cell nuclear speckle protein that regulates SPAG16 mRNA expression. PLoS One. 2011;6(5):e20625. doi: 10.1371/journal.pone.0020625. Epub 2011 May 31. PMID: 21655194; PMCID: PMC3105110.
Zhang Z, Kostetskii I, Moss SB, et al. Haploinsufficiency for the murine orthologue of Chlamydomonas PF20 disrupts spermatogenesis. Proc Natl Acad Sci U S A. 2004;101(35):12946-12951. doi:10.1073/pnas.0404280101
Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL, Moss SB, Radice GL, Strauss JF 3rd. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod. 2006 Apr;74(4):751-9. doi: 10.1095/biolreprod.105.049254. Epub 2005 Dec 28. PMID: 16382026.
SPATA31 (SPATA31 subfamily A members)
Summary
Disrupting the functioning of this sperm-specific gene has been shown to cause infertility, though its exact role is not fully understood.
The Science
Mechanistically, the loss of Spata31 in spermatids resulted in less nectin-3 and β-actin in the apical ectoplasmic specialization, indicating that SPATA31 is important for spermatid adhesion, sperm count, and male reproduction. Premature release of Spata31-/- spermatids from the seminiferous epithelium and into the epididymis has been observed, indicating that the loss of SPATA31 may reduce adhesion of spermatids to Sertoli cells via the apical ectoplasmic specialization. The apical ectoplasmic specialization maintains the polarity of elongating/elongated spermatids and confers adhesion to Sertoli cells in the seminiferous epithelium. Indeed, nectin-3 and b-actin abundance where the apical ectoplasmic specialization is normally found was significantly reduced in Spata31-/- males. Nectin-3-/- male mice are infertile, exhibit disrupted Sertoli- spermatid junctions, and lack actin filaments in their spermatids. These results are consistent with the model that β-actin, which is present in the acrosome region of spermatozoa, interacts with SPATA31 within the apical ectoplasmic specialization. SPATA31 is localized to the acrosome of round and elongated spermatids in mice. It is likely that SPATA31 functions during acrosome biogenesis and/or the acrosome reaction. However, the requirement of SPATA31 during acrosome biogenesis and/or the acrosome reactions remains unresolved.
Publications
Bekpen, C., Künzel, S., Xie, C., Eaaswarkhanth, M., Lin, Y., Gokcumen, O., . . . Tautz, D. (2017). Segmental duplications and evolutionary acquisition of UV damage response in the SPATA31 gene family of primates and humans. BMC Genomics, 18(1).
Wu YY, Yang Y, Xu YD, Yu HL. Targeted disruption of the spermatid-specific gene Spata31 causes male infertility. Mol Reprod Dev. 2015 Jun;82(6):432-40. doi: 10.1002/mrd.22491. Epub 2015 Apr 30. PMID: 25930072.
Disrupting the functioning of this sperm-specific gene has been shown to cause infertility, though its exact role is not fully understood.
The Science
Mechanistically, the loss of Spata31 in spermatids resulted in less nectin-3 and β-actin in the apical ectoplasmic specialization, indicating that SPATA31 is important for spermatid adhesion, sperm count, and male reproduction. Premature release of Spata31-/- spermatids from the seminiferous epithelium and into the epididymis has been observed, indicating that the loss of SPATA31 may reduce adhesion of spermatids to Sertoli cells via the apical ectoplasmic specialization. The apical ectoplasmic specialization maintains the polarity of elongating/elongated spermatids and confers adhesion to Sertoli cells in the seminiferous epithelium. Indeed, nectin-3 and b-actin abundance where the apical ectoplasmic specialization is normally found was significantly reduced in Spata31-/- males. Nectin-3-/- male mice are infertile, exhibit disrupted Sertoli- spermatid junctions, and lack actin filaments in their spermatids. These results are consistent with the model that β-actin, which is present in the acrosome region of spermatozoa, interacts with SPATA31 within the apical ectoplasmic specialization. SPATA31 is localized to the acrosome of round and elongated spermatids in mice. It is likely that SPATA31 functions during acrosome biogenesis and/or the acrosome reaction. However, the requirement of SPATA31 during acrosome biogenesis and/or the acrosome reactions remains unresolved.
Publications
Bekpen, C., Künzel, S., Xie, C., Eaaswarkhanth, M., Lin, Y., Gokcumen, O., . . . Tautz, D. (2017). Segmental duplications and evolutionary acquisition of UV damage response in the SPATA31 gene family of primates and humans. BMC Genomics, 18(1).
Wu YY, Yang Y, Xu YD, Yu HL. Targeted disruption of the spermatid-specific gene Spata31 causes male infertility. Mol Reprod Dev. 2015 Jun;82(6):432-40. doi: 10.1002/mrd.22491. Epub 2015 Apr 30. PMID: 25930072.
SPATA33
Summary
Disrupting the functioning of this sperm-specific gene has been shown to cause infertility, though its exact role is not fully understood.
The Science
In spermatozoa, there is a testis-enriched calcineurin composed of PPP3CC and PPP3R2 (sperm calcineurin) that is essential for sperm motility and male fertility. Because sperm calcineurin has been proposed as a target for reversible male contraceptives, identifying proteins that interact with sperm calcineurin widens the choice for developing specific inhibitors. A screen and genetic validation of calcineurin-interacting PxIxIT consensus motif showed that SPATA33 interacts with sperm calcineurin via a PQIIIT sequence. Spata33 knockout mice exhibit reduced sperm motility because of an inflexible midpiece, leading to impaired male fertility, which phenocopies Ppp3cc and Ppp3r2 knockout mice. Further analysis showed that sperm calcineurin disappears from the mitochondria in the Spata33 knockout testis.
Publications
Miyata H, Oura S, Morohoshi A, Shimada K, Mashiko D, Oyama Y, Kaneda Y, Matsumura T, Abbasi F, Ikawa M. SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2106673118. doi: 10.1073/pnas.2106673118. PMID: 34446558; PMCID: PMC8536318.
Chen H, Yi M, Sheng Y, Cheng H, Zhou R. A novel testis-enriched gene Spata33 is expressed during spermatogenesis. PLoS One. 2013 Jul 2;8(7):e67882. doi: 10.1371/journal.pone.0067882. PMID: 23844118; PMCID: PMC3699523.
Xu X, Zhang Y, Cheng H, Zhou R. SPATA33 functions as a mitophagy receptor in mammalian germline. Autophagy. 2021 May;17(5):1284-1286. doi: 10.1080/15548627.2021.1909836. Epub 2021 Apr 5. PMID: 33818286; PMCID: PMC8143240.
Disrupting the functioning of this sperm-specific gene has been shown to cause infertility, though its exact role is not fully understood.
The Science
In spermatozoa, there is a testis-enriched calcineurin composed of PPP3CC and PPP3R2 (sperm calcineurin) that is essential for sperm motility and male fertility. Because sperm calcineurin has been proposed as a target for reversible male contraceptives, identifying proteins that interact with sperm calcineurin widens the choice for developing specific inhibitors. A screen and genetic validation of calcineurin-interacting PxIxIT consensus motif showed that SPATA33 interacts with sperm calcineurin via a PQIIIT sequence. Spata33 knockout mice exhibit reduced sperm motility because of an inflexible midpiece, leading to impaired male fertility, which phenocopies Ppp3cc and Ppp3r2 knockout mice. Further analysis showed that sperm calcineurin disappears from the mitochondria in the Spata33 knockout testis.
Publications
Miyata H, Oura S, Morohoshi A, Shimada K, Mashiko D, Oyama Y, Kaneda Y, Matsumura T, Abbasi F, Ikawa M. SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2106673118. doi: 10.1073/pnas.2106673118. PMID: 34446558; PMCID: PMC8536318.
Chen H, Yi M, Sheng Y, Cheng H, Zhou R. A novel testis-enriched gene Spata33 is expressed during spermatogenesis. PLoS One. 2013 Jul 2;8(7):e67882. doi: 10.1371/journal.pone.0067882. PMID: 23844118; PMCID: PMC3699523.
Xu X, Zhang Y, Cheng H, Zhou R. SPATA33 functions as a mitophagy receptor in mammalian germline. Autophagy. 2021 May;17(5):1284-1286. doi: 10.1080/15548627.2021.1909836. Epub 2021 Apr 5. PMID: 33818286; PMCID: PMC8143240.
TEX101 (Testis Expressed Gene 101)
Summary
Involved in sperm maturation, mice without this gene create sperm that are unable to migrate to and fertilize an egg.
The Science
Tex101 is a glycosylphosphatidyl inositol (GPI)-anchored glycoprotein identified as a molecular marker of germ cells. Deletion of Tex101 affected the interactions among ADAM3, ADAM4, ADAM5, and ADAM6 in the testis, and interfered with their epididymal maturation. UTJ migration defect is the primary cause of infertility in Tex101-/- mice as Tex101-/- sperm could fertilize oocytes both in vitro and in vivo via assisted reproduction. TEX101 was found almost exclusively in the testicular germ cell extract containing up to step 8 – the spermatid stage – of spermatogenesis. Similar to CLGN, CALR3 and PDILT chaperone proteins that are not found in spermatozoa, but are required for presenting functionally active ADAM3 on sperm plasma membrane, TEX101 may also function as a ADAM-3 specific molecular chaperone.
Publications
Endo, S., Yoshitake, H., Tsukamoto, H., Matsuura, H., Kato, K., Sakuraba, M., . . . Araki, Y. (2016). TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells. Scientific Reports,6(1).
Fujihara, Y., Tokuhiro, K., Muro, Y., Kondoh, G., Araki, Y., Ikawa, M., & Okabe, M. (2013). Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proceedings of the National Academy of Sciences,110(20), 8111-8116.
Li, W., Guo, X., Teng, F., Hou, X., Lv, Z., Zhou, S., . . . Zhou, Q. (2013). Tex101 is essential for male fertility by affecting sperm migration into the oviduct in mice. Journal of Molecular Cell Biology,5(5), 345-347.
Involved in sperm maturation, mice without this gene create sperm that are unable to migrate to and fertilize an egg.
The Science
Tex101 is a glycosylphosphatidyl inositol (GPI)-anchored glycoprotein identified as a molecular marker of germ cells. Deletion of Tex101 affected the interactions among ADAM3, ADAM4, ADAM5, and ADAM6 in the testis, and interfered with their epididymal maturation. UTJ migration defect is the primary cause of infertility in Tex101-/- mice as Tex101-/- sperm could fertilize oocytes both in vitro and in vivo via assisted reproduction. TEX101 was found almost exclusively in the testicular germ cell extract containing up to step 8 – the spermatid stage – of spermatogenesis. Similar to CLGN, CALR3 and PDILT chaperone proteins that are not found in spermatozoa, but are required for presenting functionally active ADAM3 on sperm plasma membrane, TEX101 may also function as a ADAM-3 specific molecular chaperone.
Publications
Endo, S., Yoshitake, H., Tsukamoto, H., Matsuura, H., Kato, K., Sakuraba, M., . . . Araki, Y. (2016). TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells. Scientific Reports,6(1).
Fujihara, Y., Tokuhiro, K., Muro, Y., Kondoh, G., Araki, Y., Ikawa, M., & Okabe, M. (2013). Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proceedings of the National Academy of Sciences,110(20), 8111-8116.
Li, W., Guo, X., Teng, F., Hou, X., Lv, Z., Zhou, S., . . . Zhou, Q. (2013). Tex101 is essential for male fertility by affecting sperm migration into the oviduct in mice. Journal of Molecular Cell Biology,5(5), 345-347.
TRPV6 (Transient Receptor Potential Cation Channel Subfamily V Member 6)
Summary
This cation channel is believed to regulate the uptake of a calcium ion in the epididymis necessary for fertilization-ready sperm.
The Science
TRPV6 is a calcium selective cation channel that mediates Ca2+ uptake in various tissues. TRPV6 is expressed in the epididymal and prostate epithelial cells but not in sperm or germinal epithelium. The Ca2+ concentration of the fluid in the cauda epididymis of mutant Trpv6 males was 10 times higher than that of wild-type mice, which was accompanied by a seven- to eightfold decrease in Ca2+ absorption through the epididymal epithelium and was associated with reduced sperm viability. Thus, appropriate Ca2+ absorption and a consequent TRPV6-mediated decrease in the extracellular Ca2+ concentration toward the distal segments of the epididymal duct are essential for the acquisition of basic functions and the survival of spermatozoa.
Publications
Stoerger C, Flockerzi V. The transient receptor potential cation channel subfamily V member 6 (TRPV6): genetics, biochemical properties, and functions of exceptional calcium channel proteins. Biochem Cell Biol. 2014 Dec;92(6):441-8. doi: 10.1139/bcb-2014-0063. Epub 2014 Aug 26. PMID: 25372600.
Weissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, Vennekens R, Wissenbach U, Middendorff R, Flockerzi V, Freichel M. Male fertility depends on Ca²+ absorption by TRPV6 in epididymal epithelia. Sci Signal. 2011 May 3;4(171):ra27. doi: 10.1126/scisignal.2001791. PMID: 21540454.
Weissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, Mannebach S, Wissenbach U, Vennekens R, Middendorff R, Flockerzi V, Freichel M. Excision of Trpv6 gene leads to severe defects in epididymal Ca2+ absorption and male fertility much like single D541A pore mutation. J Biol Chem. 2012 May 25;287(22):17930-41. doi: 10.1074/jbc.M111.328286. Epub 2012 Mar 15. PMID: 22427671; PMCID: PMC3365704.
This cation channel is believed to regulate the uptake of a calcium ion in the epididymis necessary for fertilization-ready sperm.
The Science
TRPV6 is a calcium selective cation channel that mediates Ca2+ uptake in various tissues. TRPV6 is expressed in the epididymal and prostate epithelial cells but not in sperm or germinal epithelium. The Ca2+ concentration of the fluid in the cauda epididymis of mutant Trpv6 males was 10 times higher than that of wild-type mice, which was accompanied by a seven- to eightfold decrease in Ca2+ absorption through the epididymal epithelium and was associated with reduced sperm viability. Thus, appropriate Ca2+ absorption and a consequent TRPV6-mediated decrease in the extracellular Ca2+ concentration toward the distal segments of the epididymal duct are essential for the acquisition of basic functions and the survival of spermatozoa.
Publications
Stoerger C, Flockerzi V. The transient receptor potential cation channel subfamily V member 6 (TRPV6): genetics, biochemical properties, and functions of exceptional calcium channel proteins. Biochem Cell Biol. 2014 Dec;92(6):441-8. doi: 10.1139/bcb-2014-0063. Epub 2014 Aug 26. PMID: 25372600.
Weissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, Vennekens R, Wissenbach U, Middendorff R, Flockerzi V, Freichel M. Male fertility depends on Ca²+ absorption by TRPV6 in epididymal epithelia. Sci Signal. 2011 May 3;4(171):ra27. doi: 10.1126/scisignal.2001791. PMID: 21540454.
Weissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, Mannebach S, Wissenbach U, Vennekens R, Middendorff R, Flockerzi V, Freichel M. Excision of Trpv6 gene leads to severe defects in epididymal Ca2+ absorption and male fertility much like single D541A pore mutation. J Biol Chem. 2012 May 25;287(22):17930-41. doi: 10.1074/jbc.M111.328286. Epub 2012 Mar 15. PMID: 22427671; PMCID: PMC3365704.
Other
ADGB (Androglobin)
Summary
Increased amounts of this under-studied type of globin have been found in fertile males compared with infertile males, leading to the assumption that they are necessary for the development of healthy sperm.
The Science
Androglobins are a novel type of globin, whose function remains to be determined. The recombinantly expressed human globin domain exhibits an absorption spectrum characteristic of hexacoordination of the heme iron atom. Molecular evolutionary analyses indicate that this chimeric globin family is phylogenetically ancient and originated in the common ancestor to animals and choanoflagellates. In humans and mice, the gene is predominantly expressed in testis tissue, and the name ‘‘androglobin’’ (Adgb) has been proposed in research studies. Expression is associated with postmeiotic stages of spermatogenesis and is insensitive to experimental hypoxia. Evidence exists for increased gene expression in fertile males compared with infertile males. Spermatogenesis requires a delicate balance in its oxidative energy metabolism, between the high O2 consumption by the highly proliferative process of sperm formation and the generation of free radicals toxic to both sperm and hormone- producing testes cells. For protection, spermatogonia are equipped with a dedicated antioxidant defense. Moreover, the seminiferous tubules are avascular and O2 reaches the luminal region solely by diffusion, making this tissue hypoxic. In this environment, globin proteins, which are able either to sense O2 or to detoxify reactive oxygen species, would clearly be advantageous. The lack of hypoxic up-regulation of Adgb mRNA in vitro is in line with a sensing function, but also other roles. The Adgb globin domain could play a redox-regulated signaling function, as recently postulated for hexacoordinated vertebrate neuroglobin. Alternatively, the Adgb globin domain might mediate an O2 level-dependent protein activity, analogous to globin-coupled sensors in prokaryotes. (Learn more here.)
Publications
Hoogewijs D, Ebner B, Germani F, Hoffmann FG, Fabrizius A, Moens L, Burmester T, Dewilde S, Storz JF, Vinogradov SN, Hankeln T. Androglobin: a chimeric globin in metazoans that is preferentially expressed in Mammalian testes. Mol Biol Evol. 2012 Apr;29(4):1105-14. doi: 10.1093/molbev/msr246. Epub 2011 Nov 24. PMID: 22115833; PMCID: PMC3350324.
Santambrogio, S. & Bracke, An & Bicker, Anne & Dewilde, Sylvia & Hankeln, Thomas & Wenger, R. & Hoogewijs, David. (2015). Androglobin, the fifth mammalian oxygen-binding globin contributes to male fertility. Acta Physiologica. 213. 77-77.
Increased amounts of this under-studied type of globin have been found in fertile males compared with infertile males, leading to the assumption that they are necessary for the development of healthy sperm.
The Science
Androglobins are a novel type of globin, whose function remains to be determined. The recombinantly expressed human globin domain exhibits an absorption spectrum characteristic of hexacoordination of the heme iron atom. Molecular evolutionary analyses indicate that this chimeric globin family is phylogenetically ancient and originated in the common ancestor to animals and choanoflagellates. In humans and mice, the gene is predominantly expressed in testis tissue, and the name ‘‘androglobin’’ (Adgb) has been proposed in research studies. Expression is associated with postmeiotic stages of spermatogenesis and is insensitive to experimental hypoxia. Evidence exists for increased gene expression in fertile males compared with infertile males. Spermatogenesis requires a delicate balance in its oxidative energy metabolism, between the high O2 consumption by the highly proliferative process of sperm formation and the generation of free radicals toxic to both sperm and hormone- producing testes cells. For protection, spermatogonia are equipped with a dedicated antioxidant defense. Moreover, the seminiferous tubules are avascular and O2 reaches the luminal region solely by diffusion, making this tissue hypoxic. In this environment, globin proteins, which are able either to sense O2 or to detoxify reactive oxygen species, would clearly be advantageous. The lack of hypoxic up-regulation of Adgb mRNA in vitro is in line with a sensing function, but also other roles. The Adgb globin domain could play a redox-regulated signaling function, as recently postulated for hexacoordinated vertebrate neuroglobin. Alternatively, the Adgb globin domain might mediate an O2 level-dependent protein activity, analogous to globin-coupled sensors in prokaryotes. (Learn more here.)
Publications
Hoogewijs D, Ebner B, Germani F, Hoffmann FG, Fabrizius A, Moens L, Burmester T, Dewilde S, Storz JF, Vinogradov SN, Hankeln T. Androglobin: a chimeric globin in metazoans that is preferentially expressed in Mammalian testes. Mol Biol Evol. 2012 Apr;29(4):1105-14. doi: 10.1093/molbev/msr246. Epub 2011 Nov 24. PMID: 22115833; PMCID: PMC3350324.
Santambrogio, S. & Bracke, An & Bicker, Anne & Dewilde, Sylvia & Hankeln, Thomas & Wenger, R. & Hoogewijs, David. (2015). Androglobin, the fifth mammalian oxygen-binding globin contributes to male fertility. Acta Physiologica. 213. 77-77.
APOA1 (Apolipoprotein A1)
Summary
This protein component of cholesterol has been shown to play a role in facilitating egg fertilization.
The Science
APOAI, the major protein constituent of HDL, is a scaffold for packing phospholipids, free cholesterol, cholesterol ester, triglycerides; the protein provides thermodynamic stability and physiological functionality to HDL particles in various stages of maturation. APOA1 is known to be synthesized by the liver and intestine, and it plays a key role in reverse cholesterol transport. Recent work suggests that ovarian granulosa cells also synthesize ApoA1 following the LH surge, and SR-B1-mediated uptake is the method by which the ovary acquires cholesterol for steroidogenesis. HDL appears to be the dominant lipoprotein particle in human follicular fluid. High-density lipoprotein and ApoA1 levels in the follicular fluid are negatively associated with embryo fragmentation, suggesting that the oocyte is dependent on HDL even before fertilization and embryonic development. Apo-A1 has anti-inflammatory property, and has the ability to inhibit the synthesis of inflammatory mediators and cell adhesion molecules that might play crucial role at the time of implantation. Owing to its role in vascular biology, it is tempting to consider that aberrant endometrial secretion of this lipoprotein contributes to implantation failure.
Publications
Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, García-Velasco JA, Matorras R, Prieto B, González S, Nagore D, Simón L, Elortza F. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res. 2009 Oct;8(10):4622-32. doi: 10.1021/pr9004426. PMID: 19670903.
Gogonea V. Structural Insights into High Density Lipoprotein: Old Models and New Facts. Front Pharmacol. 2016;6:318. Published 2016 Jan 12. doi:10.3389/fphar.2015.00318
Mains LM, Christenson L, Yang B, Sparks AE, Mathur S, Van Voorhis BJ. Identification of apolipoprotein A1 in the human embryonic secretome. Fertil Steril. 2011 Aug;96(2):422-427.e2. doi: 10.1016/j.fertnstert.2011.05.049. Epub 2011 Jun 15. PMID: 21676393.
This protein component of cholesterol has been shown to play a role in facilitating egg fertilization.
The Science
APOAI, the major protein constituent of HDL, is a scaffold for packing phospholipids, free cholesterol, cholesterol ester, triglycerides; the protein provides thermodynamic stability and physiological functionality to HDL particles in various stages of maturation. APOA1 is known to be synthesized by the liver and intestine, and it plays a key role in reverse cholesterol transport. Recent work suggests that ovarian granulosa cells also synthesize ApoA1 following the LH surge, and SR-B1-mediated uptake is the method by which the ovary acquires cholesterol for steroidogenesis. HDL appears to be the dominant lipoprotein particle in human follicular fluid. High-density lipoprotein and ApoA1 levels in the follicular fluid are negatively associated with embryo fragmentation, suggesting that the oocyte is dependent on HDL even before fertilization and embryonic development. Apo-A1 has anti-inflammatory property, and has the ability to inhibit the synthesis of inflammatory mediators and cell adhesion molecules that might play crucial role at the time of implantation. Owing to its role in vascular biology, it is tempting to consider that aberrant endometrial secretion of this lipoprotein contributes to implantation failure.
Publications
Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, García-Velasco JA, Matorras R, Prieto B, González S, Nagore D, Simón L, Elortza F. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res. 2009 Oct;8(10):4622-32. doi: 10.1021/pr9004426. PMID: 19670903.
Gogonea V. Structural Insights into High Density Lipoprotein: Old Models and New Facts. Front Pharmacol. 2016;6:318. Published 2016 Jan 12. doi:10.3389/fphar.2015.00318
Mains LM, Christenson L, Yang B, Sparks AE, Mathur S, Van Voorhis BJ. Identification of apolipoprotein A1 in the human embryonic secretome. Fertil Steril. 2011 Aug;96(2):422-427.e2. doi: 10.1016/j.fertnstert.2011.05.049. Epub 2011 Jun 15. PMID: 21676393.
Gendarussa
Summary
Gendarussa is a potential male contraceptive being developed from an extract of the shrub Justicia gendarussa.
The Science
Discovered by ethnographers working with indigenous populations, Gendarussa has purportedly been in use as a contraceptive by isolated tribesmen in the Indonesian province of Papua. The mechanism of action of gendarussa is not known, and limited reports exist around how the molecule affects spermatogenesis, sperm function, or other potential means of contraception. Clinical trials in Indonesia might provide some evidence as to the feasibility of Gendarussa to be a safe, effective contraceptive for men. (Learn more here.)
Publications
Amory, John K. “Development of Novel Male Contraceptives.” Clinical and Translational Science 13, no. 2 (March 2020): 228–37. https://doi.org/10.1111/cts.12708.
Gendarussa is a potential male contraceptive being developed from an extract of the shrub Justicia gendarussa.
The Science
Discovered by ethnographers working with indigenous populations, Gendarussa has purportedly been in use as a contraceptive by isolated tribesmen in the Indonesian province of Papua. The mechanism of action of gendarussa is not known, and limited reports exist around how the molecule affects spermatogenesis, sperm function, or other potential means of contraception. Clinical trials in Indonesia might provide some evidence as to the feasibility of Gendarussa to be a safe, effective contraceptive for men. (Learn more here.)
Publications
Amory, John K. “Development of Novel Male Contraceptives.” Clinical and Translational Science 13, no. 2 (March 2020): 228–37. https://doi.org/10.1111/cts.12708.
Join Our Research Community
Are you interested in working on one of these targets? We're eager to collaborate with researchers who are passionate about advancing male contraception. Contact us to discuss potential partnerships and funding opportunities.
Are you interested in working on one of these targets? We're eager to collaborate with researchers who are passionate about advancing male contraception. Contact us to discuss potential partnerships and funding opportunities.
Expanding the Pipeline: Exploring Additional Targets
Beyond the targets listed on this page, the Contraceptive Infertility Target DataBase (CITDBase) provides a comprehensive list of human reproductive system/tissue-specific contraceptive targets. This curated database is a valuable resource for researchers seeking new and promising avenues for exploration.
Collaboration for Progress
We encourage collaboration among researchers from different areas of contraceptive research. By working together, we can accelerate progress and achieve our shared goal of Reproductive Autonomy for All.
Explore CITDBase
Visit the CITDBase to discover additional targets and potential opportunities for collaboration.
Collaboration for Progress
We encourage collaboration among researchers from different areas of contraceptive research. By working together, we can accelerate progress and achieve our shared goal of Reproductive Autonomy for All.
Explore CITDBase
Visit the CITDBase to discover additional targets and potential opportunities for collaboration.
Male Reproduction & Contraception
The science behind male reproduction can be challenging, yet it is critical to understand the biology in order to know how the male contraceptives of the future will function. In an effort to make this science more accessible, we have developed a series of primers about male reproduction and contraception: