Pharmaceutical drug development is a challenging, lengthy, and expensive process designed to ensure that the drugs we take are safe and effective.
Embark on a journey through the intricate process of drug development, from initial concept to the life-changing treatments that reach patients. Discover the stages of discovery, research, and testing that guide new medicines from the lab to your pharmacy. Learn about the challenges and triumphs faced by scientists, researchers, and advocates as they work tirelessly to ensure safety, efficacy, and accessibility. Join us in shaping the future of healthcare by understanding the remarkable path of drug development. Together, let's embrace the mission of improving lives through innovation.
A Comprehensive Approach to Male Contraception Research
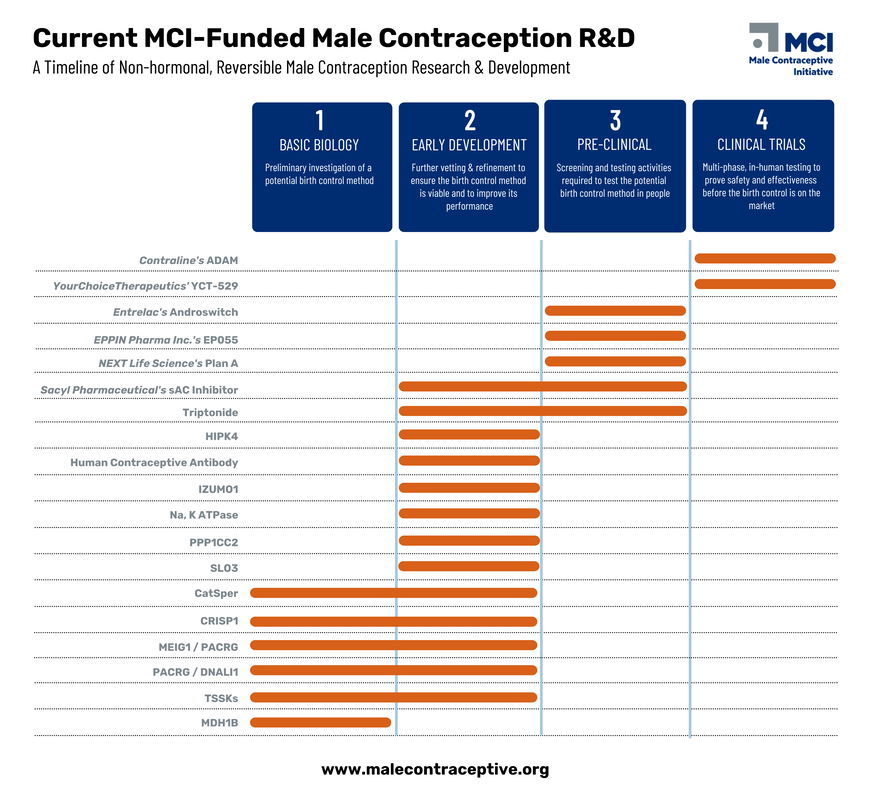
At Male Contraceptive Initiative, we support a wide range of research projects across the entire product development pipeline. Our grantees are working on innovative solutions at every stage, from early-stage method investigation to advanced clinical trials.
We're always seeking promising research projects to support. Explore our current initiatives and learn how you can contribute to the future of male contraception:
We're always seeking promising research projects to support. Explore our current initiatives and learn how you can contribute to the future of male contraception:
Navigating the complex process of evaluating and testing new drugs and therapeutic interventions can be challenging. This primer provides a simplified overview to help you understand the key stages involved.
- Discovery and Development: Identifying potential drug candidates and conducting initial research.
- Preclinical Testing: Evaluating safety and efficacy in animals.
- Clinical Trials: Testing the drug in human subjects through multiple phases.
- Regulatory Review: Submitting the drug for approval by regulatory agencies like the FDA.
- Post-Market Surveillance: Monitoring the drug's safety and effectiveness after approval.
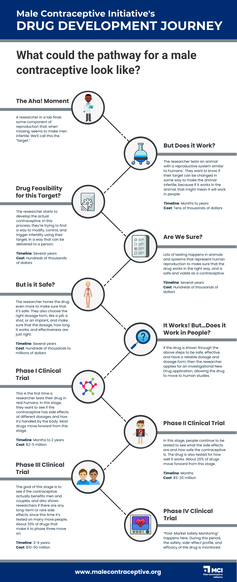
The Drug Development Process
Pharmaceutical drug development is a meticulously orchestrated process that transforms scientific concepts into life-changing medications, improving human health and well-being. This intricate journey involves a myriad of stages, rigorous testing, and regulatory oversight to ensure safety, efficacy, and quality. In this exploration, we'll delve into how drugs are researched, designed, and tested before they become available to the public. (To see where non-hormonal male contraceptives supported by MCI are in the development process, please click here.)
|
Stage 1: Discovery and Research
The journey begins with a spark of inspiration, often stemming from a new understanding of diseases, mechanisms, or unmet medical needs. Researchers embark on a quest to identify potential drug candidates that could address these challenges. This involves extensive literature reviews, laboratory experiments, and computer simulations to explore possible molecular targets and pathways. Once a potential target is identified – usually a specific protein or biological process involved in a disease – scientists work on designing molecules that can interact with this target to produce a desired effect. |
Stage 2: Preclinical Development
Promising molecules move from the theoretical realm to the laboratory bench. In preclinical development, these molecules are rigorously tested in vitro (in cells) and in vivo (in animals) to assess their safety, pharmacokinetics (how the body processes the drug), and potential efficacy. Researchers evaluate factors such as toxicity, potential side effects, and dosing regimens, while optimizing the chemical structure of molecules to enhance effectiveness and reduce the potential for side effects. Data collected during preclinical testing provide the basis for selecting the most promising candidate drug(s) to move forward. |
|
Stage 3: Clinical Trials
The transition from preclinical development to clinical trials is a monumental leap. Clinical trials are the cornerstone of drug development, providing evidence of a drug's safety and efficacy in humans. The process is divided into several phases:
|
Stage 4: Post-Approval Monitoring
Even after a drug receives regulatory approval and enters the market, the journey doesn't end. Post-approval monitoring is essential to detect any previously unforeseen side effects or long-term impacts. Pharmaceutical companies, regulatory agencies, and healthcare providers collaborate to collect real-world data and monitor the drug's safety and effectiveness in a larger patient population. MCI's "The Drug Development Pipeline"
|
MCI's "Drug Development Pathway" Infographic
Challenges in Drug Development, Generally
The path from a groundbreaking scientific idea to a life-changing medication is fraught with challenges that test the limits of innovation, patience, and resources. The process of pharmaceutical drug development is a complex interplay of scientific discovery, regulatory scrutiny, and the pursuit of improving global health.
In this section, we explore the formidable obstacles that researchers, developers, and regulators face in this intricate endeavor.
In this section, we explore the formidable obstacles that researchers, developers, and regulators face in this intricate endeavor.
Overcoming Challenges for a Healthier Future
Researchers, pharmaceutical companies, and regulatory agencies demonstrate their unwavering commitment to patient health and well-being by navigating the complex challenges of drug development. Each hurdle serves as an opportunity for growth, adaptation, and advancements in medical science. The journey from discovery to market may be arduous, but the ultimate goal of bringing safe and effective medications to those in need remains a powerful driving force for innovation in male contraception research and development.
Researchers, pharmaceutical companies, and regulatory agencies demonstrate their unwavering commitment to patient health and well-being by navigating the complex challenges of drug development. Each hurdle serves as an opportunity for growth, adaptation, and advancements in medical science. The journey from discovery to market may be arduous, but the ultimate goal of bringing safe and effective medications to those in need remains a powerful driving force for innovation in male contraception research and development.
Specific Challenges in Developing Non-Hormonal, Reversible Male Contraceptives
The pursuit of non-hormonal, reversible male contraceptives presents a distinct set of challenges within the realm of pharmaceutical drug development. As researchers and advocates strive to expand the options for family planning and reproductive health, they encounter obstacles that demand innovative solutions and unwavering commitment. Let's delve into the unique challenges that arise in this groundbreaking field.
MCI's "Development Challenges for Male Contraceptives" Infographic
Despite the challenges, researchers, advocates, and stakeholders remain steadfast in their commitment to advancing non-hormonal, reversible male contraceptives. Each obstacle serves as an opportunity to drive innovation, promote inclusivity, and reshape the landscape of family planning options.
By overcoming these challenges, we can bring safe, effective, and flexible contraceptive choices to couples worldwide. This underscores the importance of ongoing research and support in this crucial area of reproductive health.
By overcoming these challenges, we can bring safe, effective, and flexible contraceptive choices to couples worldwide. This underscores the importance of ongoing research and support in this crucial area of reproductive health.
Advocacy & Funding
|
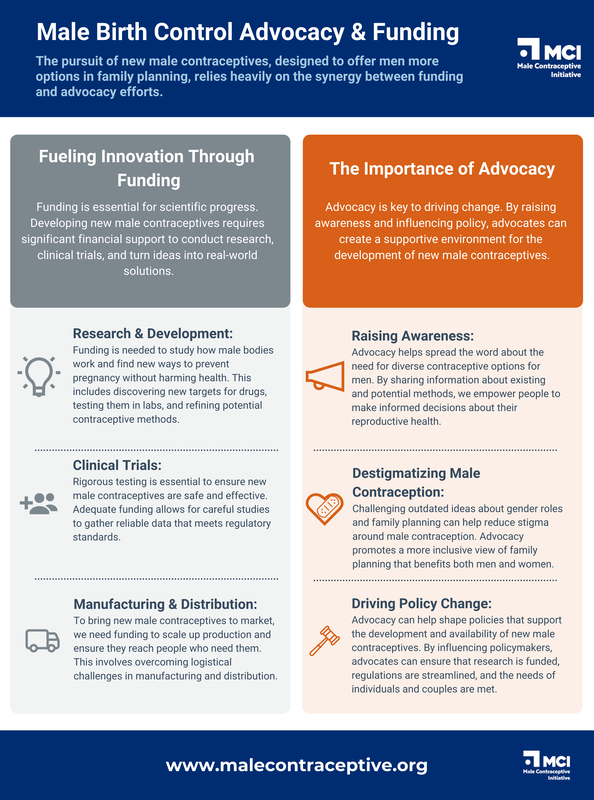
The development of new male contraceptives hinges on a strong foundation of funding and advocacy. These two pillars work together to:
Together, funding and advocacy are critical for driving progress and ensuring that men have the same range of contraceptive options as women. |
MCI's "Advocating for Male Contraception" Animated Short
MCI's "Contraceptive Funding, Simplified" Animated Short
|
|
MCI's "Male Birth Control Advocacy & Funding" Infographic
|
The development of new male contraceptives is a collaborative journey that requires a strong foundation of funding and advocacy. While researchers work tirelessly to unlock scientific breakthroughs, advocates play a crucial role in ensuring that these advancements are recognized, prioritized, and integrated into the broader conversation about reproductive health.
By investing in funding and supporting advocacy efforts, individuals, organizations, and governments can contribute to a future where couples have a wider range of contraceptive options. Through this collective commitment, we can transform the landscape of family planning, embracing innovation and inclusion to empower individuals and couples in shaping their reproductive futures. Learn More about MCI's Funding Strategy Visit our Funding page to learn more about Male Contraceptive Initiative's approach to funding research and development in male contraception: |
Be a Catalyst for Change: Join the Fight for Global Health
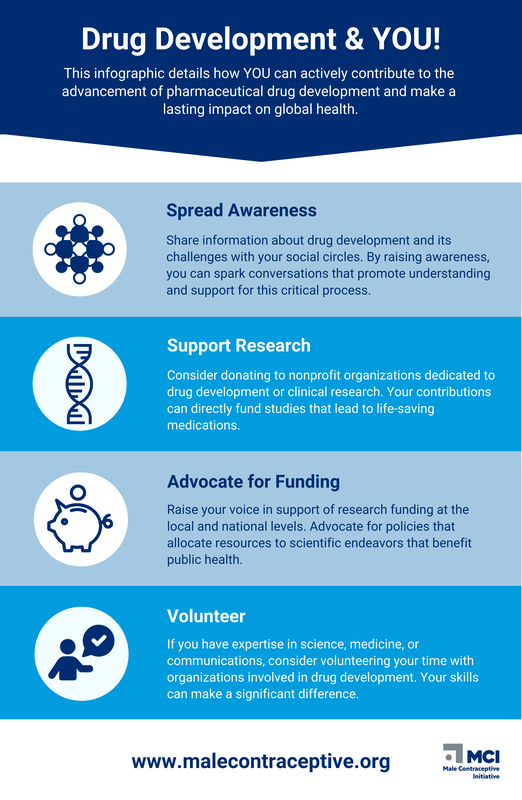
You have the power to make a lasting impact on global health by contributing to the advancement of pharmaceutical drug development. Your involvement could be instrumental in bringing life-saving treatments to millions around the world.
Here's how you can get involved:
Together, we can create a healthier future for generations to come.
Here's how you can get involved:
- Support research: Donate to fund groundbreaking research projects.
- Advocate for change: Raise awareness about the importance of drug development and advocate for policies that support innovation.
- Volunteer your time: Contribute to our efforts through volunteer work or mentorship.
- Spread the word: Share our mission with your friends, family, and community.
Together, we can create a healthier future for generations to come.
MCI's "Drug Development & YOU" Infographic
By getting involved, you become a catalyst for progress and innovation in the field of pharmaceutical drug development. Your actions can pave the way for safer, more effective medications that impact lives on a global scale.
Join the Movement:
Learn More
Visit our Resources page to discover how you can add your voice to the cause of "Reproductive Autonomy for All":
Join the Movement:
- Donate: Support our research and advocacy efforts.
- Volunteer: Contribute your time and skills to our cause.
- Advocate: Spread awareness and promote policies that support drug development.
- Partner: Collaborate with us to create a lasting impact.
Learn More
Visit our Resources page to discover how you can add your voice to the cause of "Reproductive Autonomy for All":
Frequently Asked Questions (FAQs)
About us
Male Contraceptive Initiative (MCI) is a 501(c)3 nonprofit whose objective is to advocate for and promote the development of reversible non-hormonal male contraception. MCI was founded in 2014 by a group of interested individuals who saw a philanthropic model as an innovative method to promote the development of male contraceptives. Since then, we have grown in scope and capacity, funding over $5M in research activities since our inception. Our Executive Board comprises experts in medicine, research, contraceptive development, and marketing, and we retain key advisors and stakeholders in the contraceptive research space.
MCI’s vision is “Reproductive Autonomy for All” and our mission is “To empower men, and couples, to fully contribute to family planning goals by providing them the resources they need for reproductive autonomy.”
We fund scientific studies, support early-stage researchers, and engage with the public on why male contraception will yield an advanced and as yet, unimagined modern landscape that:
We are funded through private donations from people like you. Join us on our journey to make “Reproductive Autonomy for All” a reality!
MCI’s vision is “Reproductive Autonomy for All” and our mission is “To empower men, and couples, to fully contribute to family planning goals by providing them the resources they need for reproductive autonomy.”
We fund scientific studies, support early-stage researchers, and engage with the public on why male contraception will yield an advanced and as yet, unimagined modern landscape that:
- Provides men with an option that is safe and reversible;
- Increases access and contraceptive choices for all;
- Enables informed choice about family growth and planning;
- Offers a complete menu of options for contraception;
- Fosters shared responsibility between spouses and partners;
- Reduces stress on the climate and environment; and
- Provides a solution for a heretofore unaddressed need
We are funded through private donations from people like you. Join us on our journey to make “Reproductive Autonomy for All” a reality!
What sort of products are in development?
Male contraceptives of the future can take a number of forms, with a wide range of product characteristics. This could be a daily pill, an implant, or even a coitally-dependent method that is taken only just before sex.
We like to think of male contraceptives in development as coming in three categories.
We like to think of male contraceptives in development as coming in three categories.
- Vas-occlusive devices. These can be likened to a vasectomy, and block the passage of sperm through the vas deferens. Unlike a vasectomy, these devices are intended to be reversible, and act long-term.
- Drug-based non-hormonal male contraception. This area is a very broad topic, and includes multiple targets with multiple mechanisms of action. Products could take the form of pills, implants, gels, or other modes of delivery. Depending on a number of factors, non-hormonal male contraceptives could potentially be short-acting, or quite long-acting, and there are many projects in development that span these diverse potential product characteristics. MCI focuses our funds on methods like vas-occlusive devices and drug-based non-hormonal approaches.
- Hormonal male contraception. This method uses administration of exogenous hormones to shut down sperm production, and is in active development with governmental and other support.
When will it hit the market?
Science has intended to get male contraceptives on the market for decades. Of the products currently in development, current estimates are 15-20 years away for a drug-based product and 5-10 years away for a device.
Listen to Intended, a new podcast from us where we discuss male contraception--learning from the past, talking to the researchers of the present, and the users of the future.
Listen to Intended, a new podcast from us where we discuss male contraception--learning from the past, talking to the researchers of the present, and the users of the future.
Why do you only support non-hormonal efforts?
Hormonal male contraceptives are currently in clinical trials, with support from the NIH and other funders. Non-hormonal male contraceptives are comparatively underfunded, despite offering a greater opportunity for innovation and methods that meet the needs of more users. MCI supports non-hormonal, early-stage research efforts, investing in the long-term to create an eventual menu of options for men and women that meet their lifestyle and individual needs. We believe that all methods of contraception are meaningful components of a continuum of reproductive care necessary to provide everyone with the autonomy to decide what’s best for them and when.
What happens with the research you fund?
MCI strives for transparency in all our efforts. Our grantees publish their results as Open Access so that the broader community can learn about the latest advances in male contraception. Consistent with our global mission, we also promote access to the eventual products for communities worldwide.
Will men actually use it?
|
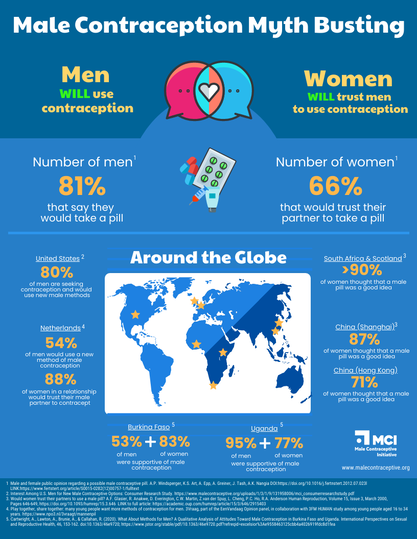
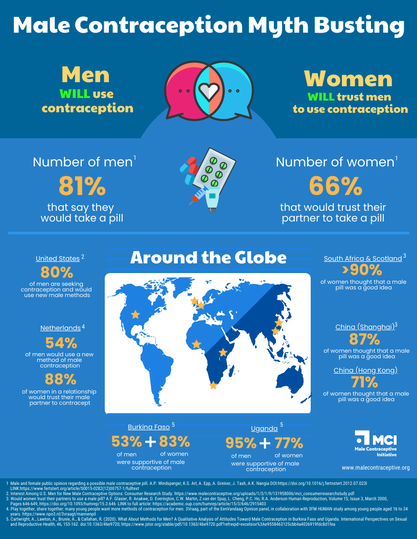
There are multiple studies that show the majority of men are interested in male contraceptives, and want the ability to use them. MCI’s own research suggests a potential market of 17 million men in the United States alone. And that’s now – before there are any methods on the market at all. Once it becomes a normal conversation for men to be involved in contraception, we expect that more and more men will be interested.
|
Male Contraception Myth Busting
|
Will women trust men to use it?
|
Male contraceptives will allow new options for couples who find the current landscape doesn’t meet their needs. In surveys, most women would trust their partner to use male contraceptives. Male contraceptives will add to the method mix, providing new options that allow men and women to contribute in whatever way they deem appropriate to contraceptive use. Those who wish to continue using current forms of contraception will still have that option, and each situation will be unique to the individual.
|
Male Contraception Myth Busting
|
Why has it taken so long?
|
The unfortunate saying goes – “Male contraception has been 10 years away for the past 50 years.” There are many reasons for these delays including scientific hurdles, financing, and public interest. However, the field is more exciting than it’s ever been. Research is progressing down previously unexplored pathways with new technological advances, and an increasingly vocal contingent of men and women are highlighting the need for new options.
Our podcast Intended investigates the past, present, and future of male contraception. Season 1 is currently available and its episodes provide details as to why we don’t yet have contraceptive methods for men beyond the condom and vasectomy. |
Drug Development Pathway
|
What are the biggest hurdles to making a male contraceptive?
First and foremost, funding. It can cost well over a billion dollars to get a drug all the way onto the market, and in an age where funding is scarce, male contraception rarely makes it to the top of the priority list. MCI pushes projects further along the pipeline to de-risk them and provide incentive for other funders to participate in the landscape.
Secondly, the regulatory pathway for male contraceptives is uncertain. These products will be unlike any other, and the steps required to ensure their safety and efficacy are yet-to-be determined. Male Contraceptive Initiative works with researchers and other stakeholders to clarify that regulatory path, and ask the important questions early. Other hurdles include the scientific challenges inherent in making a safe, reversible contraceptive that meets high clinical standards, and eventual policy issues in making it available for the consumer.
Secondly, the regulatory pathway for male contraceptives is uncertain. These products will be unlike any other, and the steps required to ensure their safety and efficacy are yet-to-be determined. Male Contraceptive Initiative works with researchers and other stakeholders to clarify that regulatory path, and ask the important questions early. Other hurdles include the scientific challenges inherent in making a safe, reversible contraceptive that meets high clinical standards, and eventual policy issues in making it available for the consumer.
What do you think about hormonal male contraception?
We’re very optimistic about hormonal male contraceptive trials, as they will allow men to participate in the family planning process in a way they haven’t been able to before. These eventual products will also create data about what men use, need, and enjoy about contraception, and shape how we guide the innovative products of the future.
Will male contraceptives offer STI / STD prevention?
Simply due to technology, first-generation male contraceptive products aren’t expected to offer sexually-transmitted infection / disease (STI) prevention. Multipurpose prevention technology (MPT) products are a class of products currently being researched to deliver contraception and varied combinations of HIV prevention and other STI prevention. We’re hopeful that innovative future products for men and women will find ways to incorporate these features.
Will women continue using their own contraception?
Many women use hormonal contraception for reasons other than preventing pregnancy. This is an individual choice for women to make on a case-by-case basis, considering their contraceptive needs, relationship, and other factors. We view contraception not as an “either, or” situation, but rather an “and” one: everyone should have access to the contraceptive methods they want and need. It is to everyone’s benefit that people have the tools and resources necessary to achieve their reproductive autonomy.
What are the differences between creating male and female methods?
|
There are lots of differences between developing male and female contraceptive options. Existing female contraceptives generally rely on using active pharmaceutical ingredients (APIs) that have already been approved by the Food and Drug Administration (FDA). This makes their approval and release easier, since much more is known about the safety and efficacy of the drug. Male options are yet to be developed, especially non-hormonal contraceptives. These drugs will need to be fully tested and vetted before they are allowed on the market.
In addition, biology is quite different. Female hormonal contraceptives generally work by preventing ovulation, thickening cervical mucus, and thinning the lining of the uterus. Male contraceptives will need to target the creation or maturation of sperm, it’s transport out of the male reproductive system, or it’s ability to swim to and fertilize an egg. These drastically different biological functions are complex, and need in-depth scientific study to understand how we might block them in a targeted, non-systemic way. |
Existing Contraceptive Methods
|
How can I learn more about conferences and other events around the male contraception space?
We regularly update our Events page to provide you listings of local and international conferences and meetings. This page also gives you access to brief event descriptions along with logistical details. You can sign up for our newsletter to receive information on upcoming events.
Does Male Contraceptive Initiative have published works?
Male Contraceptive Initiative has completed several projects that have led to publication of scientific literature and in general media. Many of these studies and reports are self-published. We also highlight scientific articles that our grantees have published. These resources can be found on our Publications page.
Is there a place where scientific terminology and/or phrases are defined and explained further?
The Glossary provides you quick access to a collection of defined scientific terms and phrases. It also includes words from popular culture in reference to the male reproductive system. You can also visit our videos and webinars, as well as our blog for more in depth explorations of these concepts and terminologies.
Where can I sign up?
You can sign up for our newsletter below for updates on MCI. We’ll notify you of new ways you can participate in the creation of methods for men as we have them.